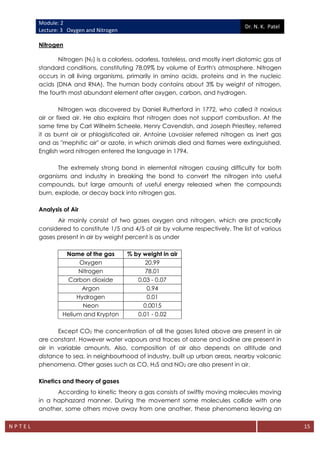
The document discusses the properties and significance of oxygen and nitrogen, the primary components of Earth's atmosphere, along with their roles in living organisms and industrial processes. It explains the methods of oxygen and nitrogen production, including the Linde and Claude processes, detailing the distillation and liquefaction techniques employed to separate these gases from air. Additionally, it covers the kinetic theory of gases and outlines the critical temperatures and pressures necessary for the liquefaction of various gases.