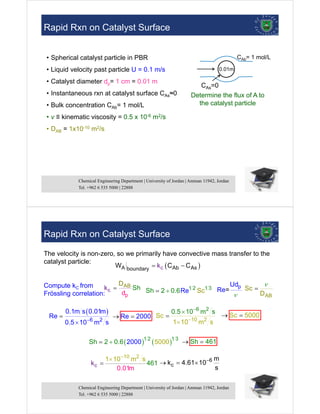
The document is a lecture on heterogeneous catalysis, focusing on external diffusion effects and mass transfer in packed beds. It discusses the importance of transport processes in the design of chemical reactors, emphasizes the complexities of rate equations in multi-phase reactions, and details the principles of mass transfer, including bulk motion and diffusion. Various examples, boundary conditions, and calculations related to mass transfer resistance and flux evaluation are also provided.