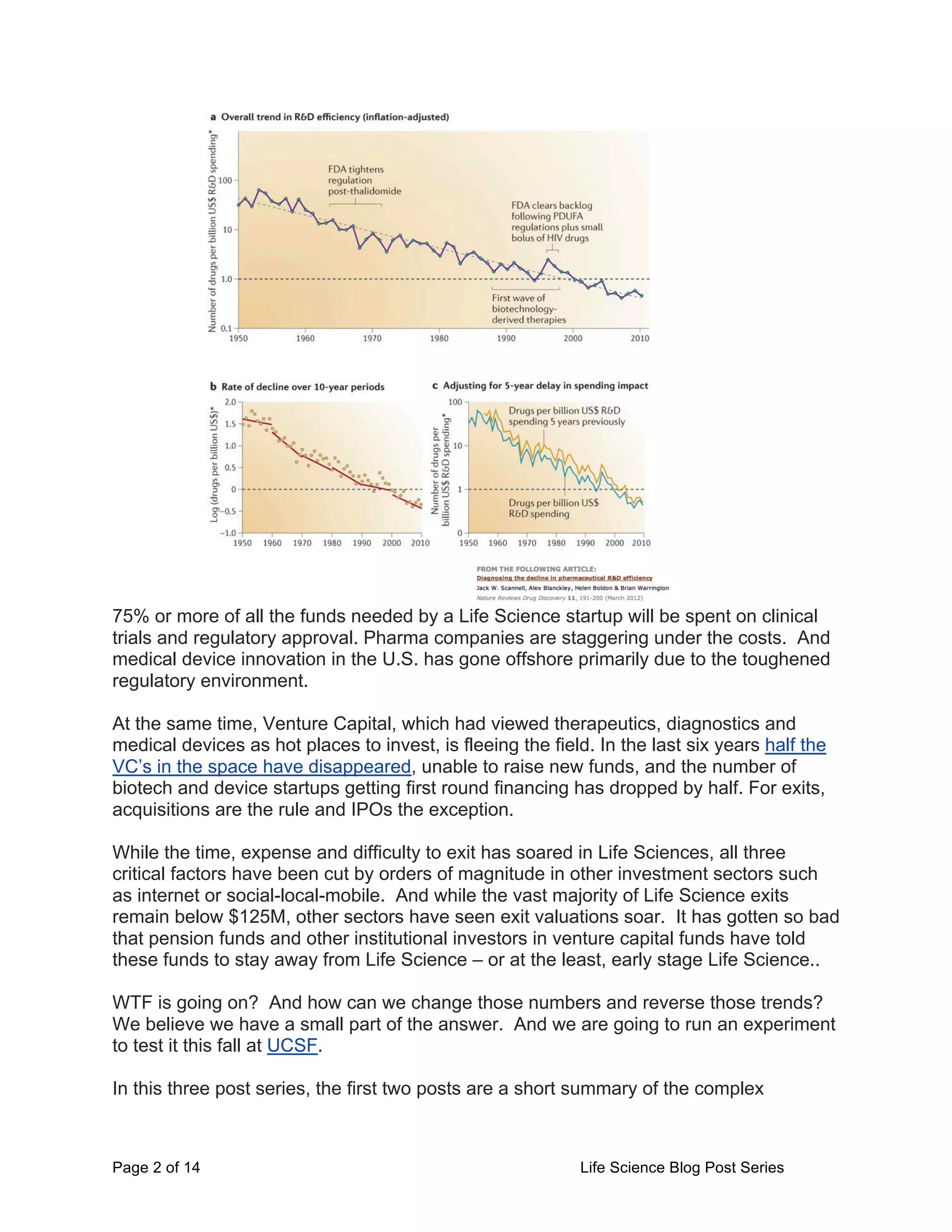
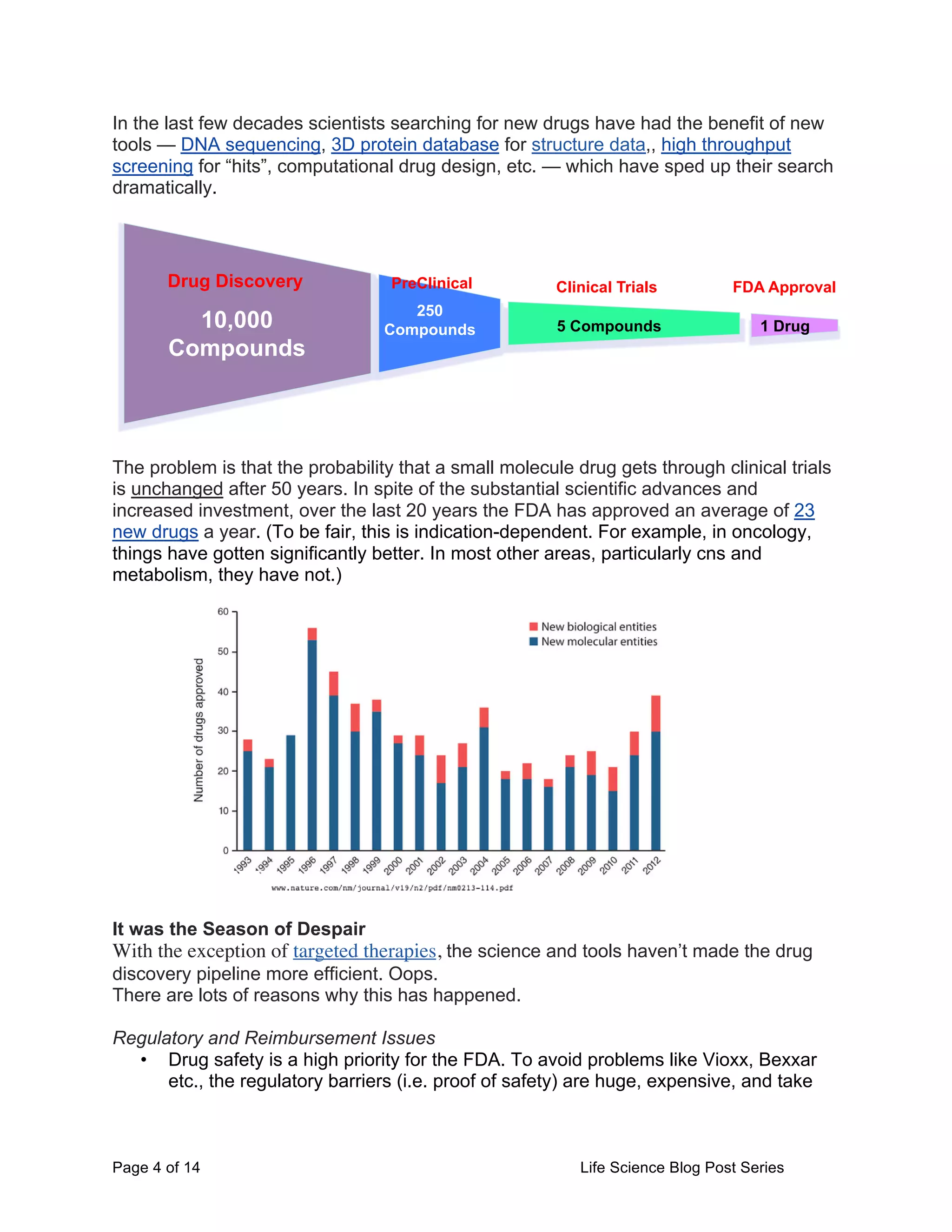
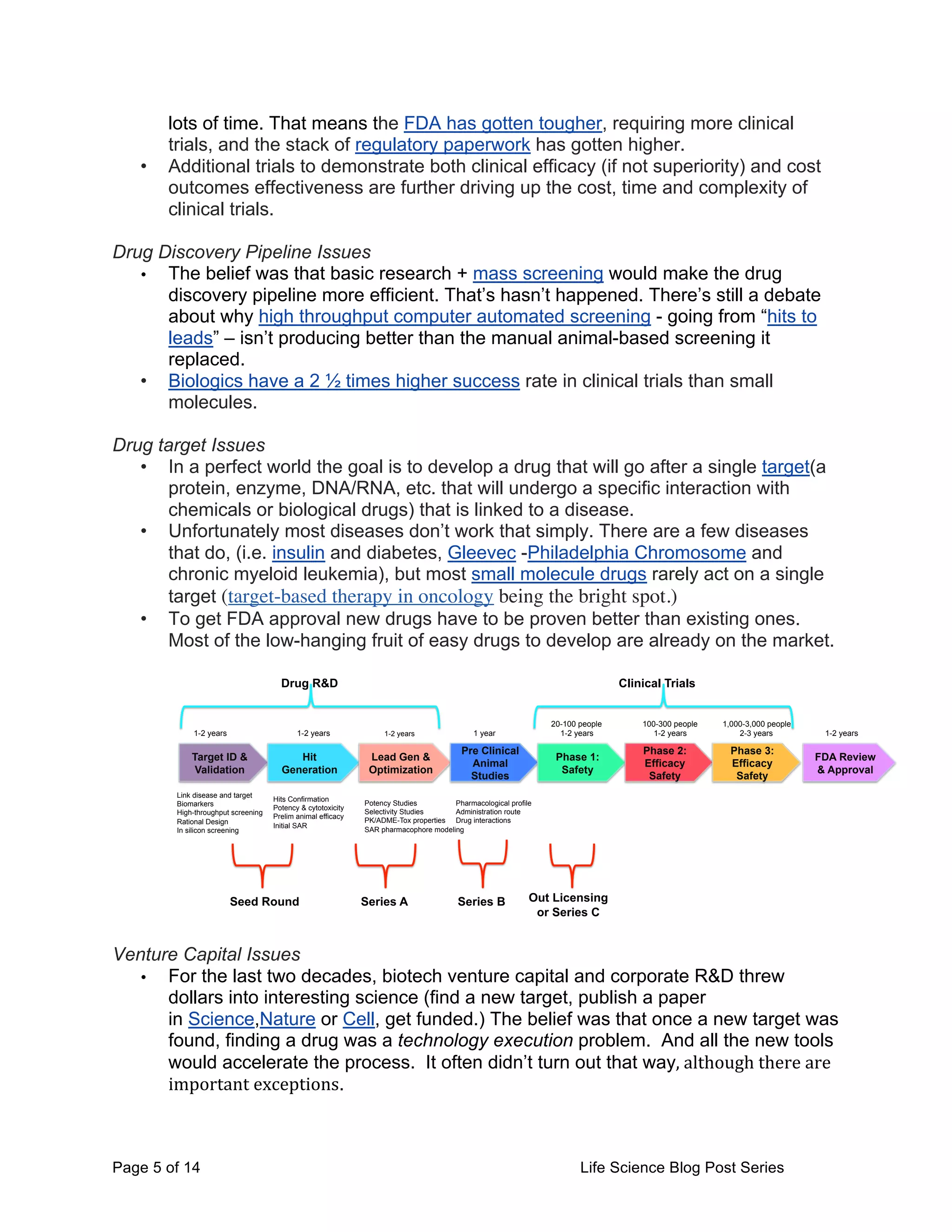
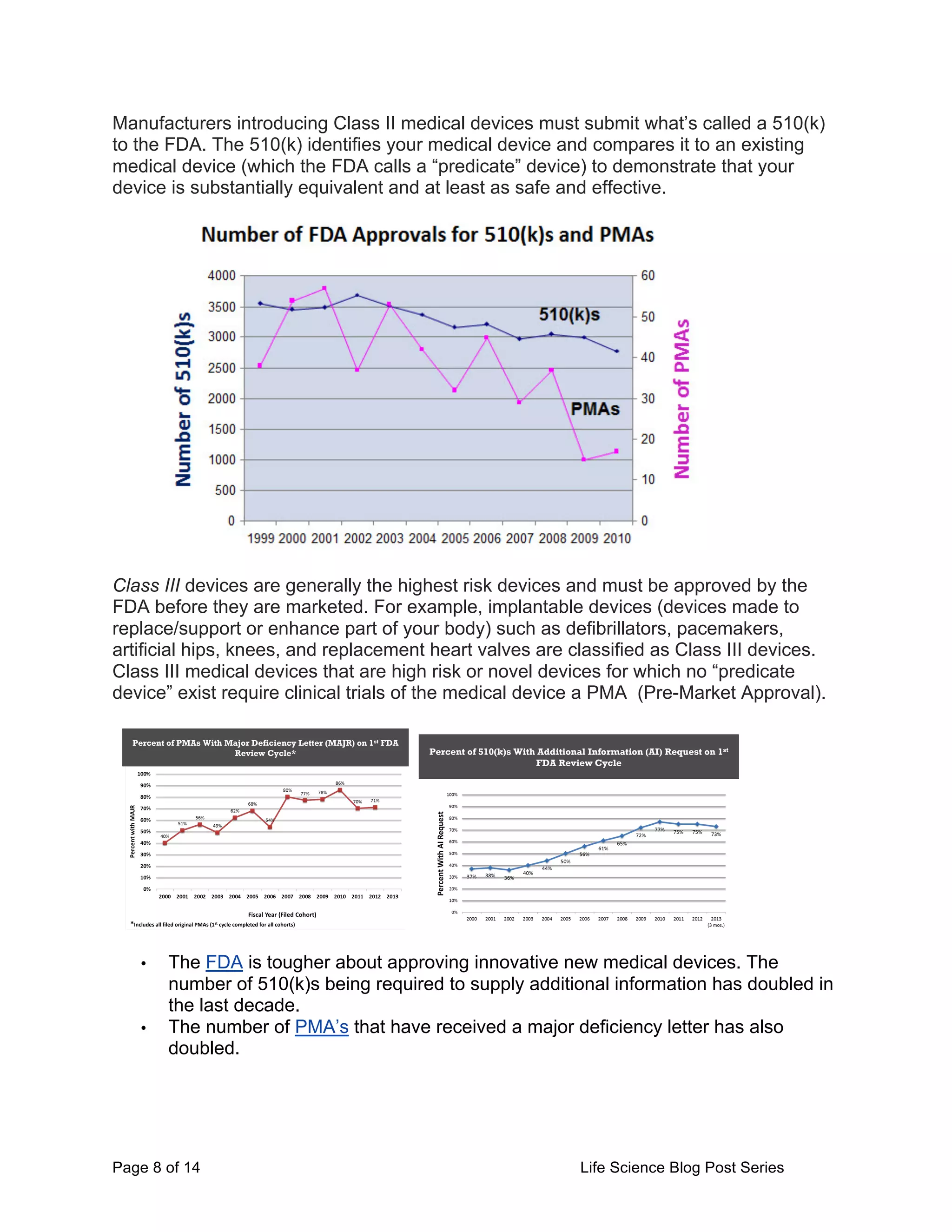
This document provides an overview of the challenges facing life science startups in therapeutics, diagnostics, medical devices, and digital health. It discusses the increasing costs and regulatory hurdles of drug and device development, including higher clinical trial standards and FDA scrutiny. This has led to declining venture capital investment in life sciences as startups face greater challenges in achieving approval and profitability. The author proposes testing a Lean LaunchPad approach to help life science startups commercialize research more efficiently and reverse negative industry trends.