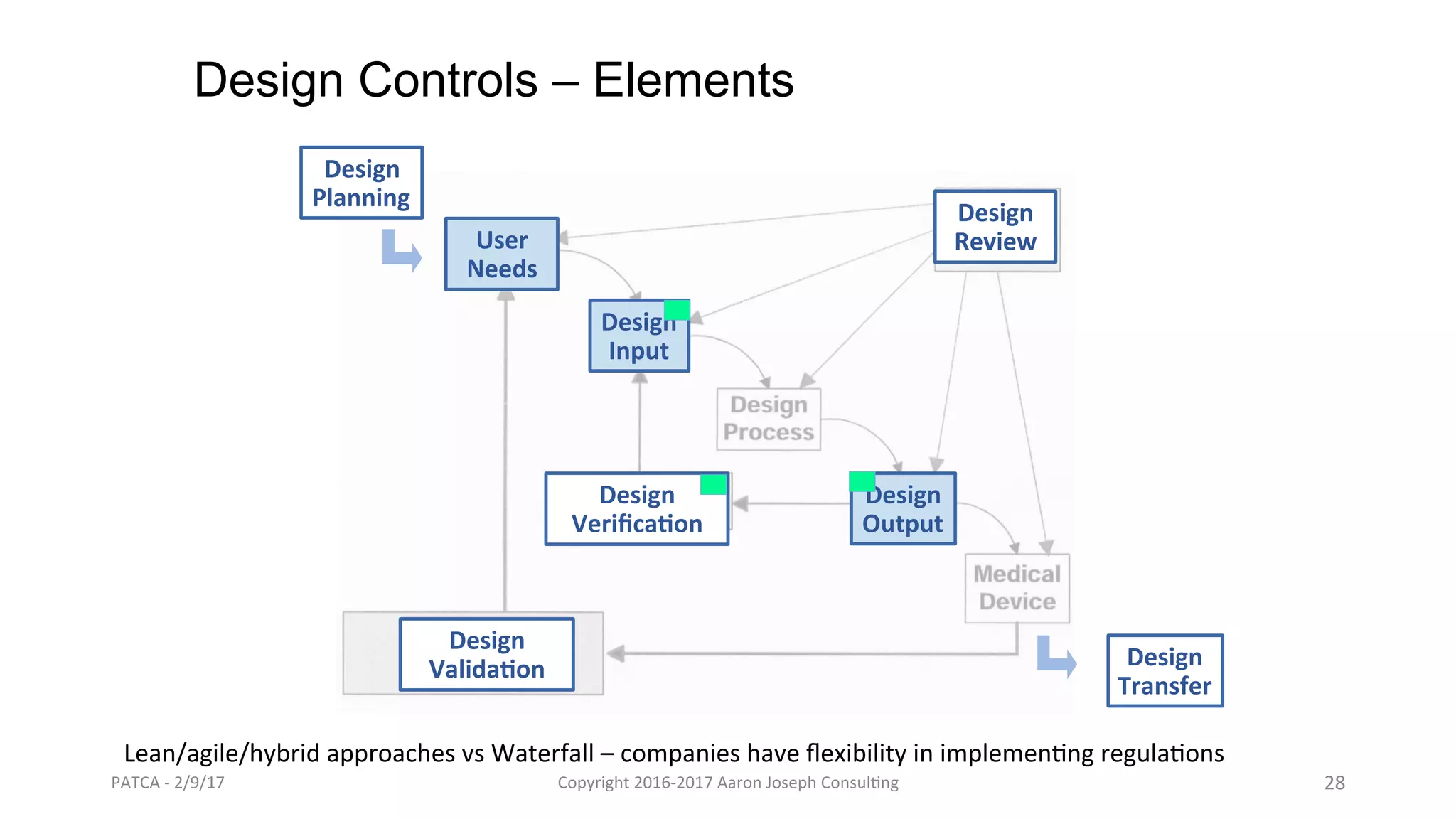
The document discusses leveraging lean and agile methods to enhance medical device development, addressing common issues such as design flaws and compliance challenges. It compares traditional project approaches to lean-agile methods through real-life examples and highlights the importance of knowledge management and iterative testing in regulatory environments. It advocates for adapting existing quality systems to support these agile methodologies while ensuring compliance with industry regulations.
















![The Agile Manifesto (2001)
We are uncovering beDer ways of developing soRware by doing it and helping
others do it. Through this work we have come to value:
Individuals and interac.ons over processes and tools
Working so]ware over comprehensive documentaVon
Customer collabora.on over contract negoVaVon
Responding to change over following a plan
That is, while there is value in the items on the right,
we value the items on the leR more.
Feb. 2001 - hDp://agilemanifesto.org/
Medical Device RegulaVons è processes & documentaVon
SoluVon: find a balance to maintain benefits of Agile while sVll fulfilling regulatory requirements
AAMI TIR45:2012 – Guidance on Use of Agile for Medical Device So]ware
PATCA - 2/9/17 Copyright 2016-2017 Aaron Joseph ConsulVng 17](https://image.slidesharecdn.com/lean-agilefeb2017patcaajosephss-170210172349/75/Lean-agile-feb2017-patca_a_joseph_ss-17-2048.jpg)

![Waterfalls
FDA Design Controls (21CFR820.30)
IEC 62304 Medical Device So]ware Standard
5.1 SW Development Planning
5.2 SW
Requirements
Analysis
5.3 SW
Architectural
Design
5.4 SW
Detailed
Design
5.5 SW Unit
ImplementaVon
& VerificaVon
5.6 SW
IntegraVon &
IntegraVon TesVng
5.7 SW
System
TesVng
5.8 SW
Release
PATCA - 2/9/17 Copyright 2016-2017 Aaron Joseph ConsulVng 19](https://image.slidesharecdn.com/lean-agilefeb2017patcaajosephss-170210172349/75/Lean-agile-feb2017-patca_a_joseph_ss-19-2048.jpg)
![5.1 SW Development Planning
5.2 SW
Requirements
Analysis
5.3 SW
Architectural
Design
5.4 SW
Detailed
Design
5.5 SW Unit
ImplementaVon
& VerificaVon
5.6 SW
IntegraVon &
IntegraVon TesVng
5.7 SW
System
TesVng
5.8 SW
Release
7. Risk
Mgmt
7. Risk
Mgmt
7. Risk
Mgmt
Transposing Waterfall to Agile (IEC 62304 Medical SW Standard)
5.1 SW Development Planning (sprint planning)
5.2 SW Requirements Analysis
5.3 SW Architectural Design
5.4 SW Detailed Design
5.5 SW Unit ImplementaVon & VerificaVon
5.6 SW IntegraVon & IntegraVon TesVng
5.7 SW System TesVng & Regression TesVng
5.8 SW Release
For each Sprint
AAMI TIR45:2012 – Guidance on Use of Agile for Medical Device So]ware
For each Release
5.7 SW System
TesVng
5.1 SW Development Planning (release planning)
For each Project 5.1 SW Development Planning (project)
5.2 SW Requirements Analysis (high-level, backlog mgmt)
5.3 SW Architectural Design (infrastructure)
5.6 SW
IntegraVon &
IntegraVon TesVng
PATCA - 2/9/17 Copyright 2016-2017 Aaron Joseph ConsulVng 20](https://image.slidesharecdn.com/lean-agilefeb2017patcaajosephss-170210172349/75/Lean-agile-feb2017-patca_a_joseph_ss-20-2048.jpg)