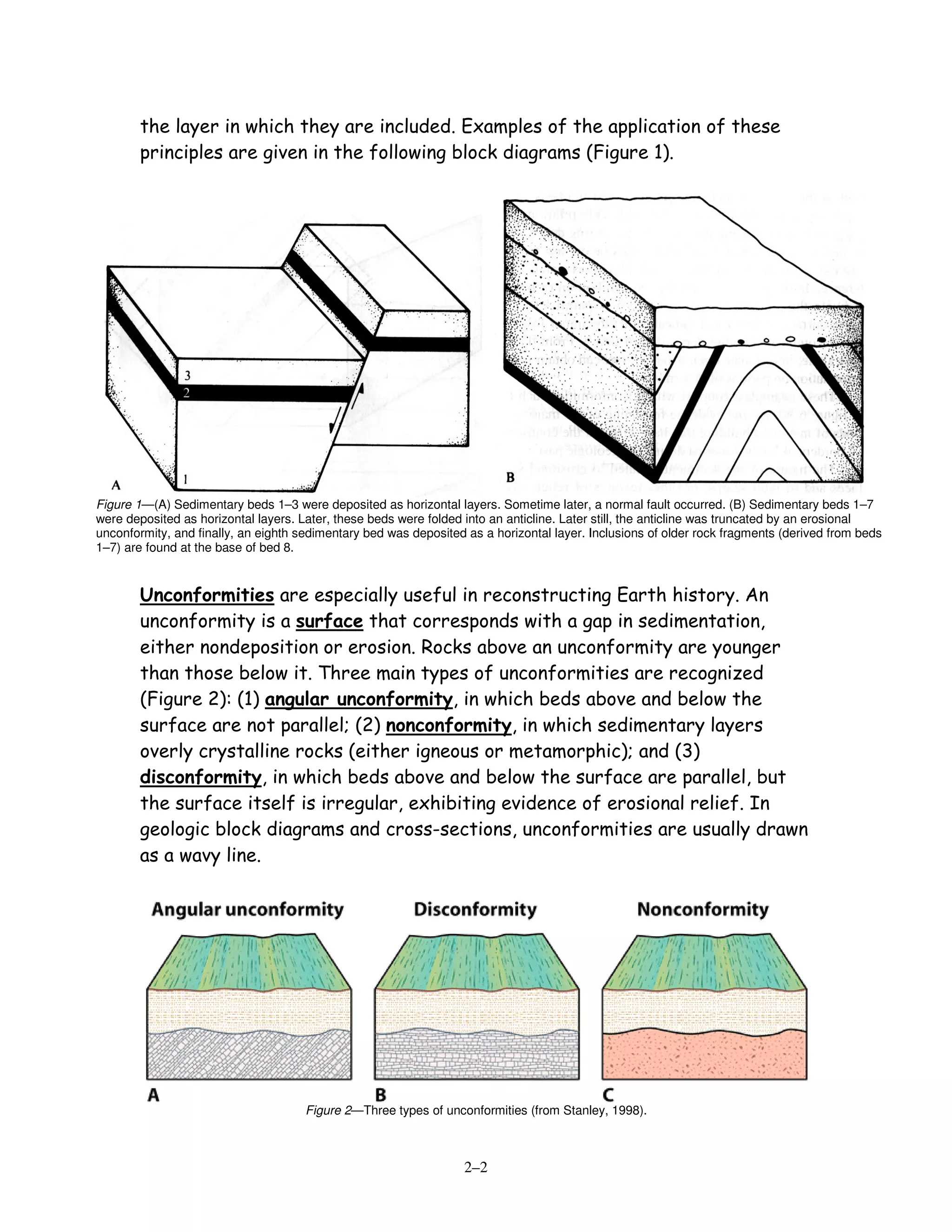
This document discusses principles of relative and absolute dating of geologic events.
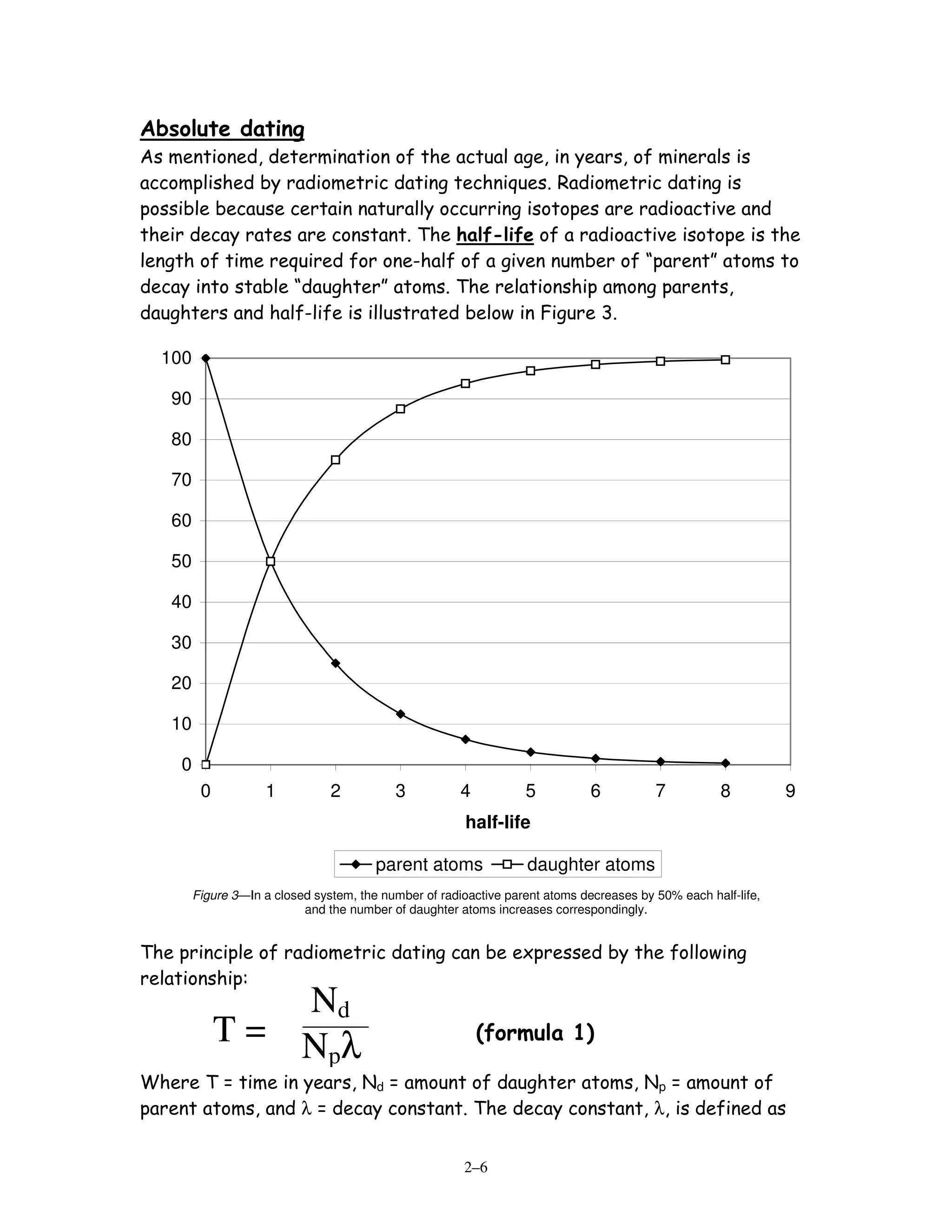
[1] Relative dating involves determining the sequence of events without knowing exact ages, by applying principles like superposition, cross-cutting relationships, and inclusion. Absolute dating determines exact ages in years using radiometric dating techniques that rely on radioactive decay of isotopes like carbon-14 and uranium-238.
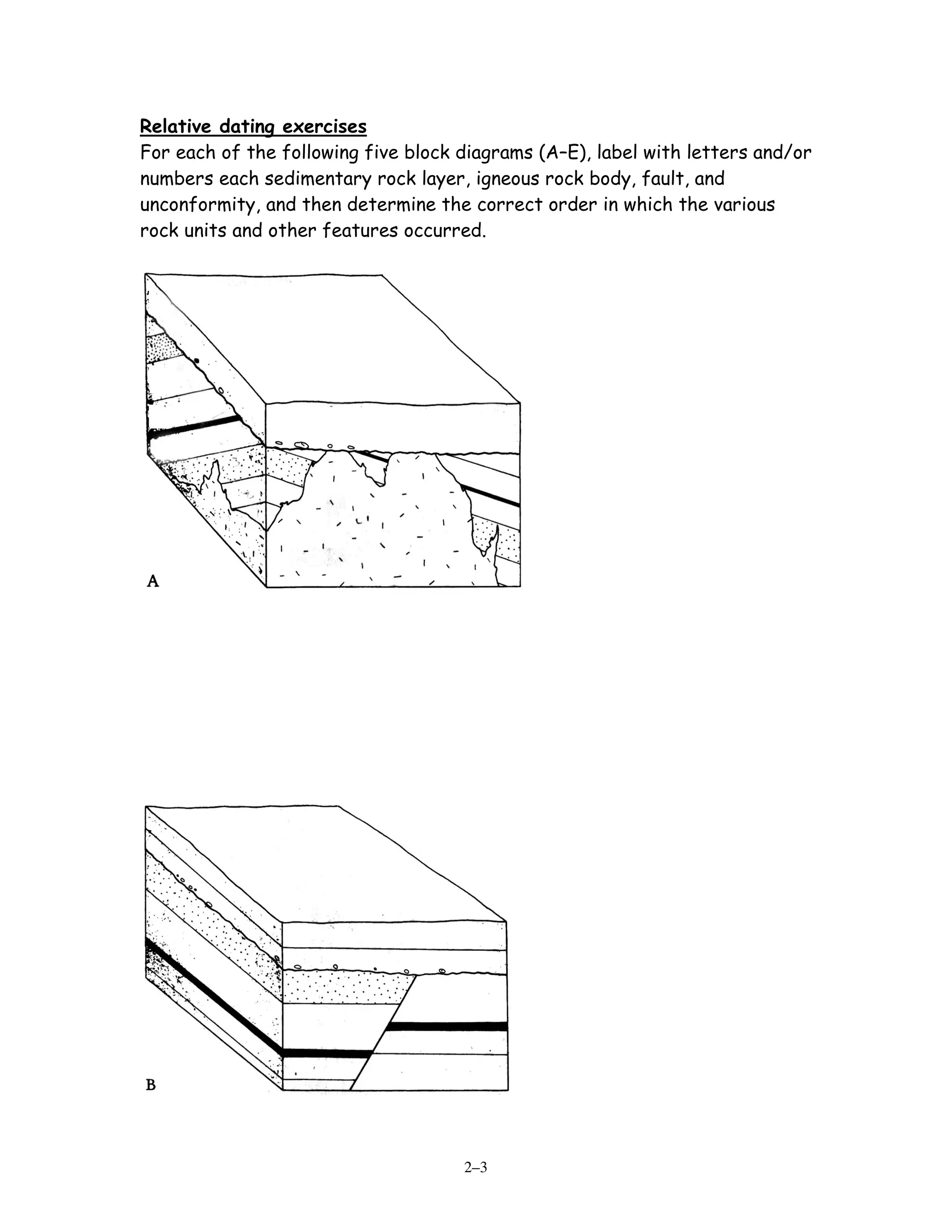
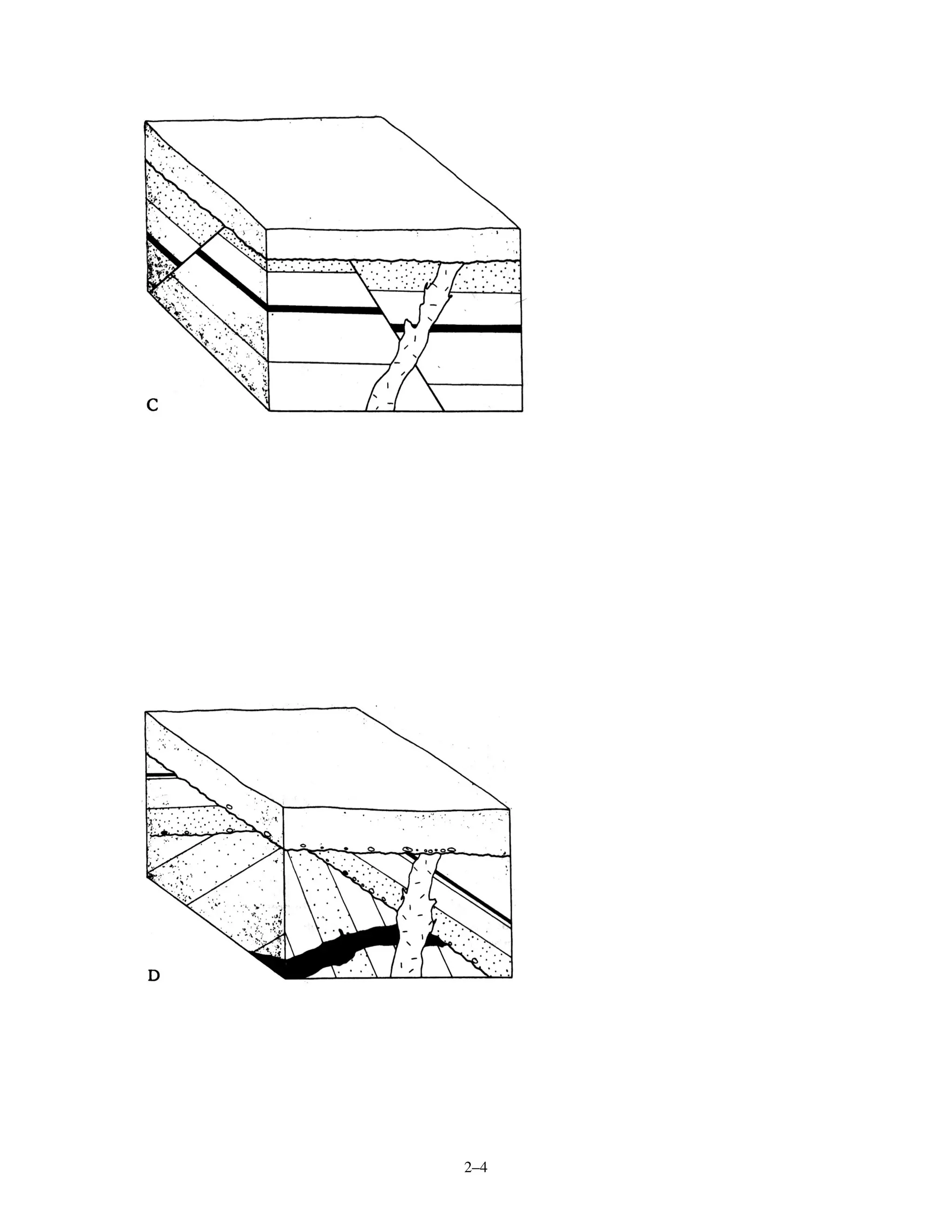
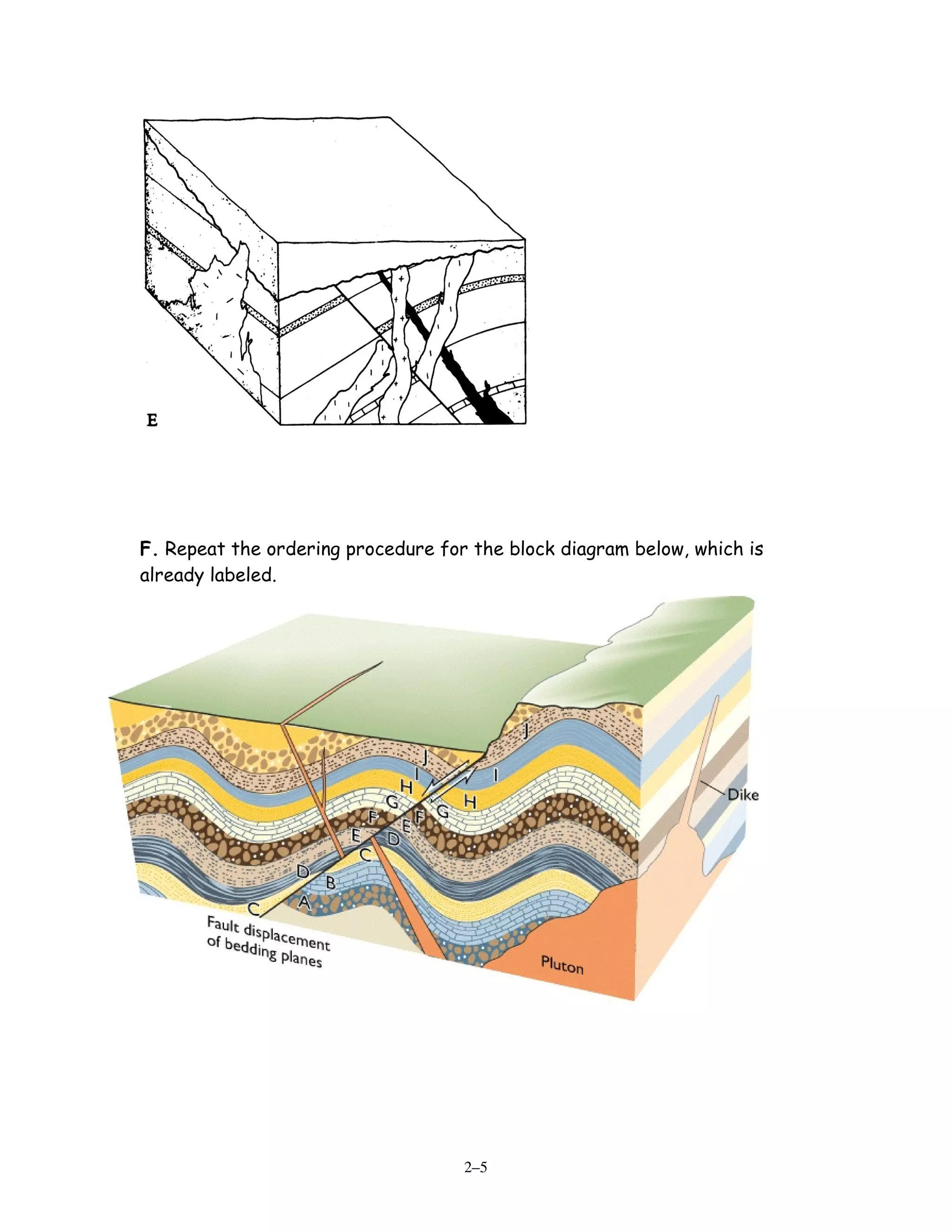
[2] Five diagrams are presented and labeled for practice determining the sequence of rock layers, faults, and unconformities using relative dating principles. Radiometric dating equations are also shown relating radioactive parents and stable daughter isotopes to calculate the time since a rock formed.

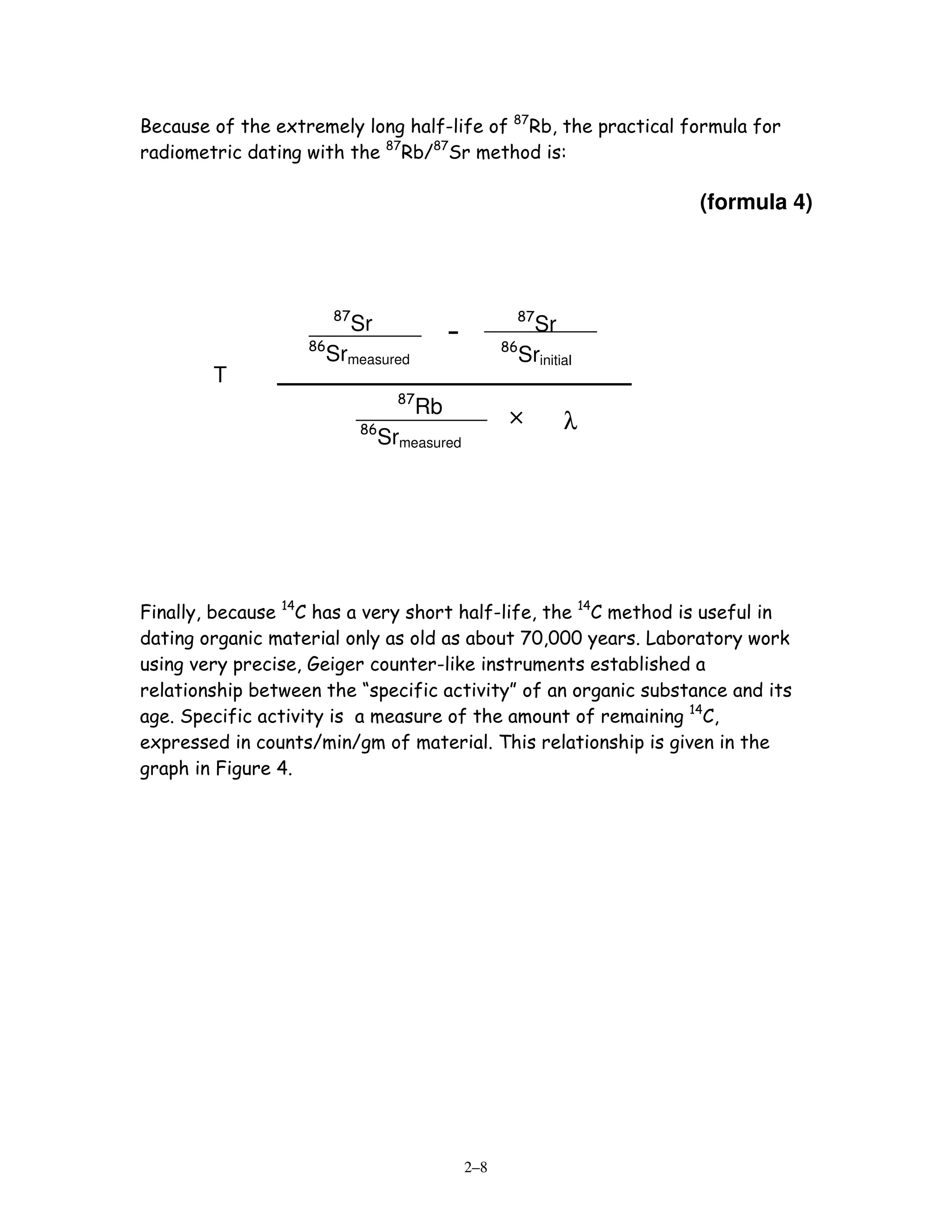
![0.693/half-life. Table 1 lists some commonly used radioactive parents /
daughters, their half-lives, λ’s, and effective dating ranges.
Table 1—Isotopes commonly used in radiometric dating.
Isotopes Half-life λ Effective dating
range
238
U / 206Pb 4.46 × 109 years 1.55 × 10-10 > 100 m.y.
40
K / 40Ar 1.25 × 109 years λε = 0.581 × 10-10 > 100,000 years
λβ = 4.96 × 10-10
87
Rb / 87Sr 4.88 × 1010 years 1.42 × 10-11 > 100 m.y.
14
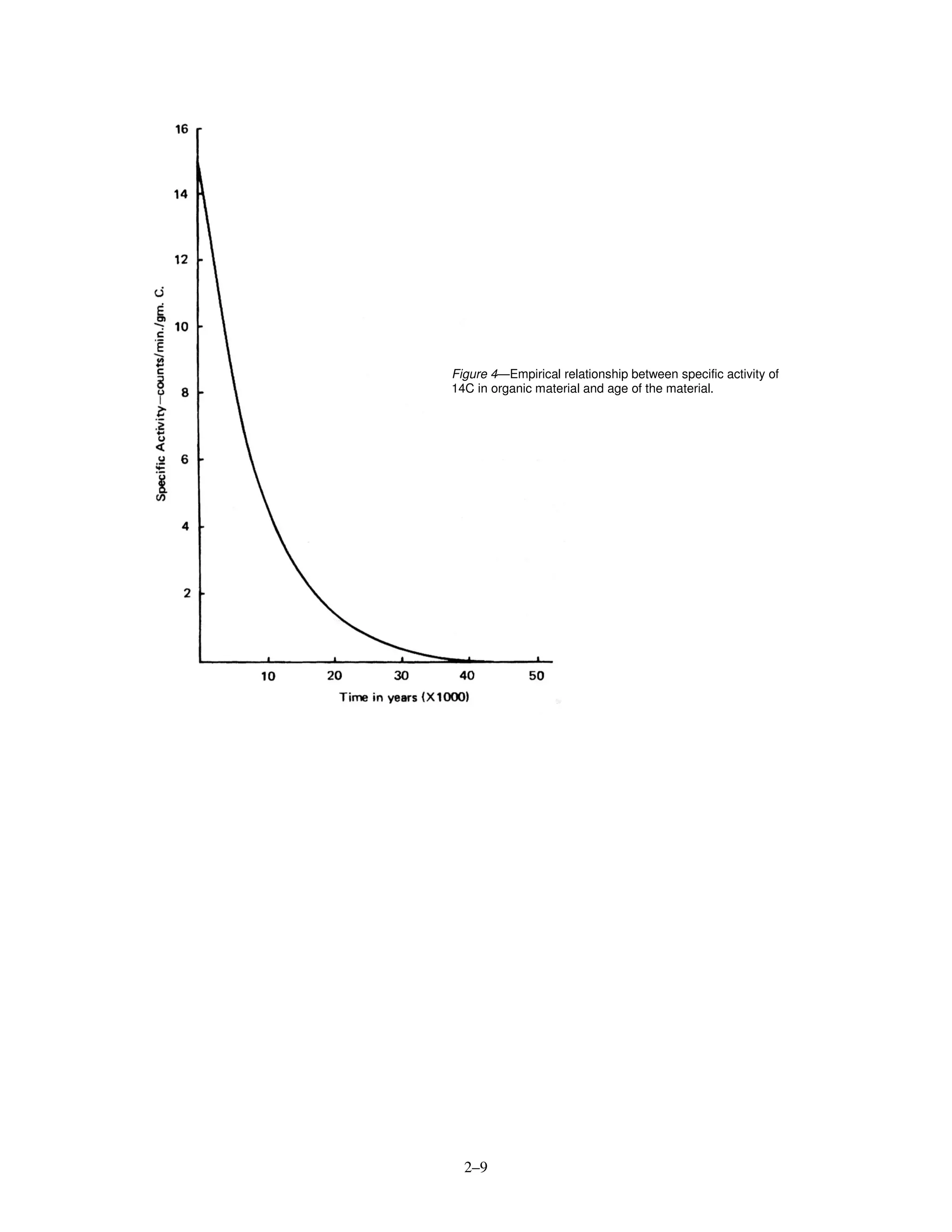
C 5730 years ~50,000–70,000
years
Radiometric dates reflect the time that has elapsed since a mineral formed
and its chemical composition was set. Because the dating procedure requires
measuring the existing amounts of parent and daughter isotopes, it is
critically important to analyze only those mineral grains that have remained
closed systems since their time of origin. A key requirement in radiometric
dating is that there has been no loss or gain of parent or daughter atoms
through partial melting, metamorphism, weathering, or any other agent.
Another requirement in radiometric dating is that no daughter atoms were
originally present in the grain to be analyzed. If some daughter atoms were
originally present, then they must be corrected for.
For 238U/206Pb dating, the general relationship in formula 1 (above) can be
rewritten as follows:
(Dp – Di)
T=
1
λ
× loge [ Pp +1 ] (formula 2)
Where Dp = present amount of daughter
Di = initial amount of daughter
Pp = present amount of parent
T = time in years since crystallization of the grain
Formula 2 must be modified for 40K/40Ar dating, because 40K decays into two
daughter products, 40Ca and 40Ar. The modified equation is:
T=
1
(λε + λβ)
× loge [[ 40
40
Ar
K
×
λε + λβ
λε
] +1 ] (formula 3)
2–7](https://image.slidesharecdn.com/labexercise02-101202032024-phpapp02/75/Lab-exercise02-7-2048.jpg)