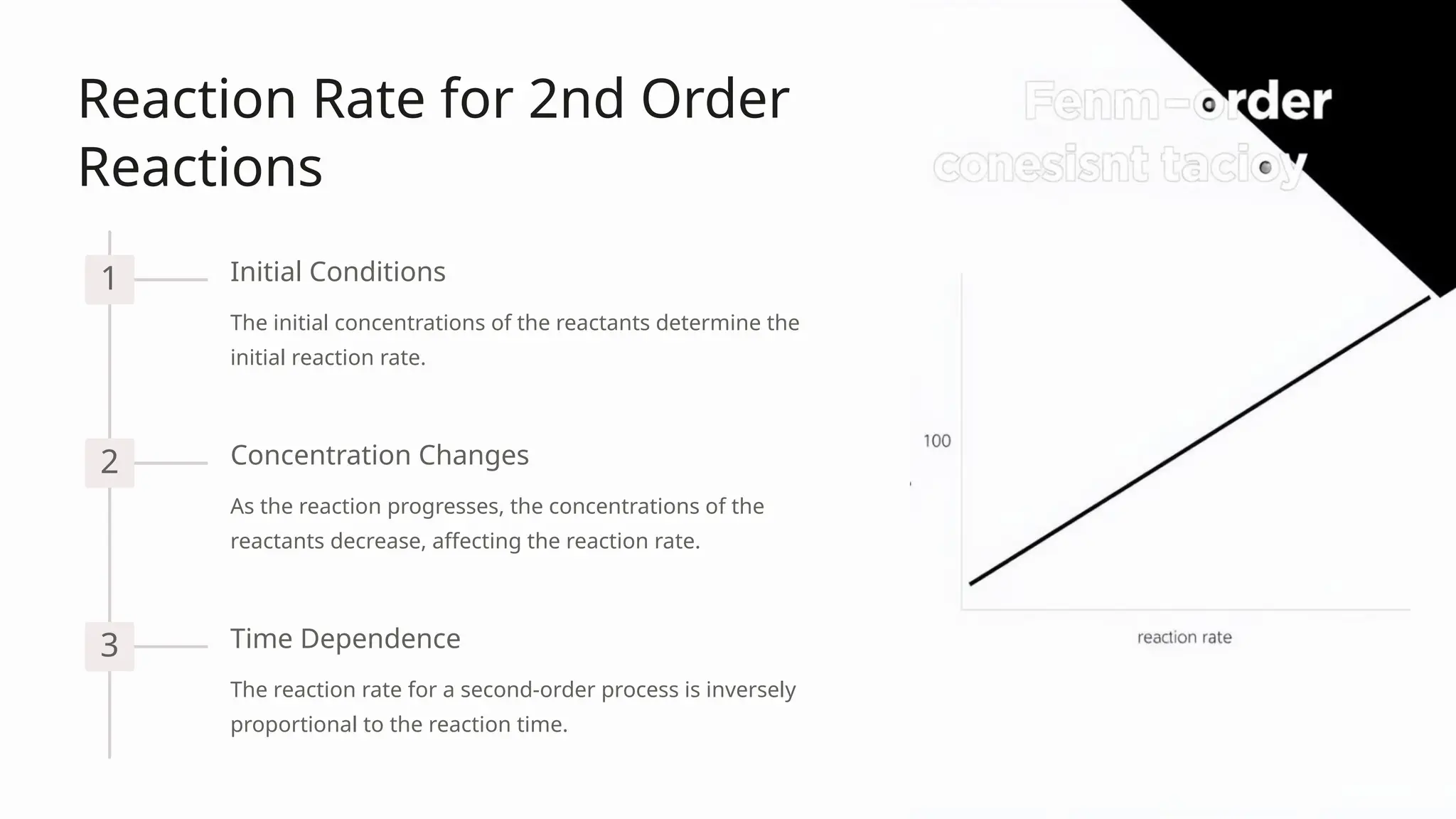
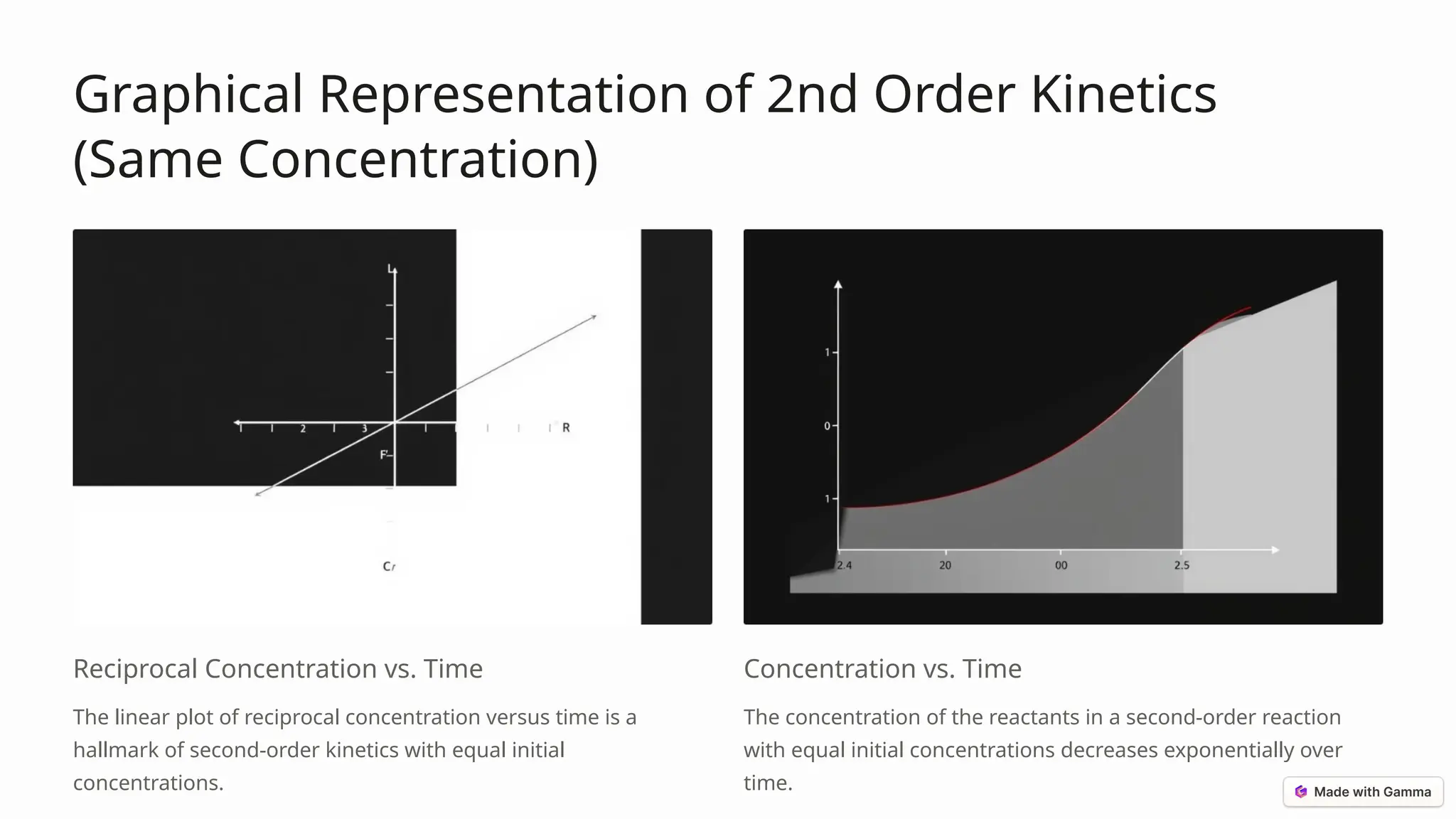
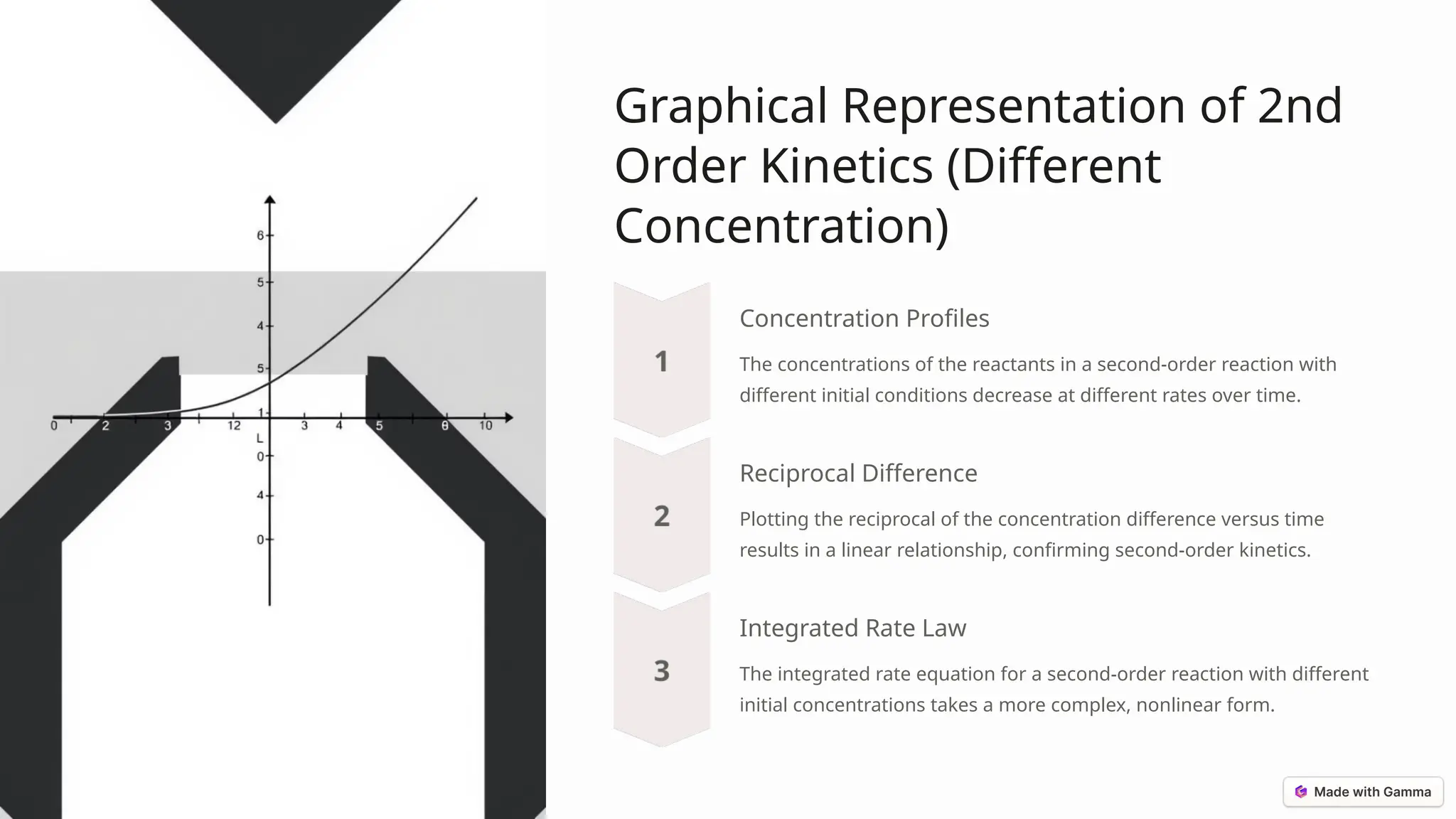
The document discusses second-order reactions, detailing the relationship between reaction rates and reactant concentrations, and emphasizing the importance of understanding reaction dynamics and mechanisms. It covers the derivation of the rate equations, graphical representations, and how initial conditions affect reaction behavior, as well as practical applications in organic synthesis, enzyme kinetics, and atmospheric chemistry. Key takeaways highlight the significance of second-order kinetics in various fields of chemistry and engineering.