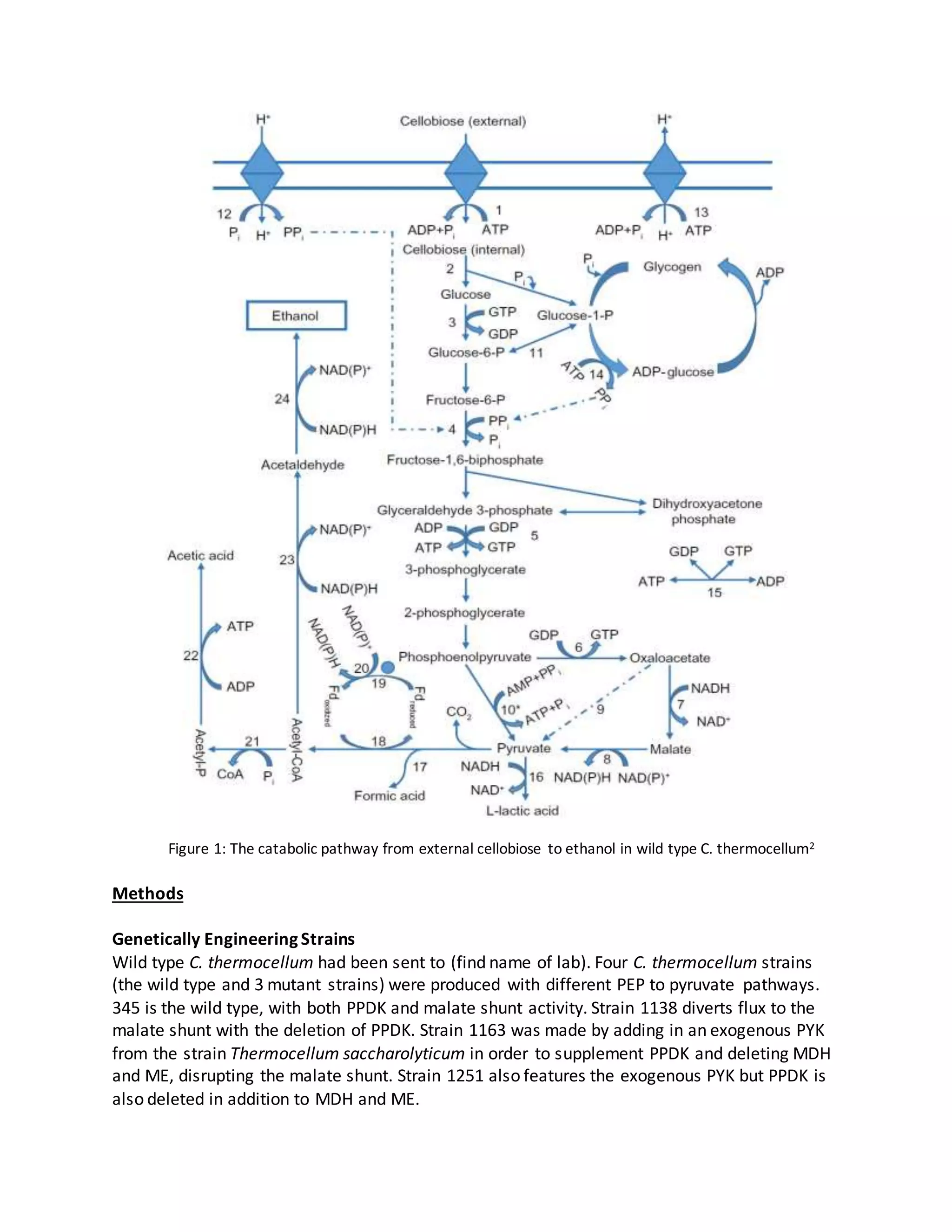
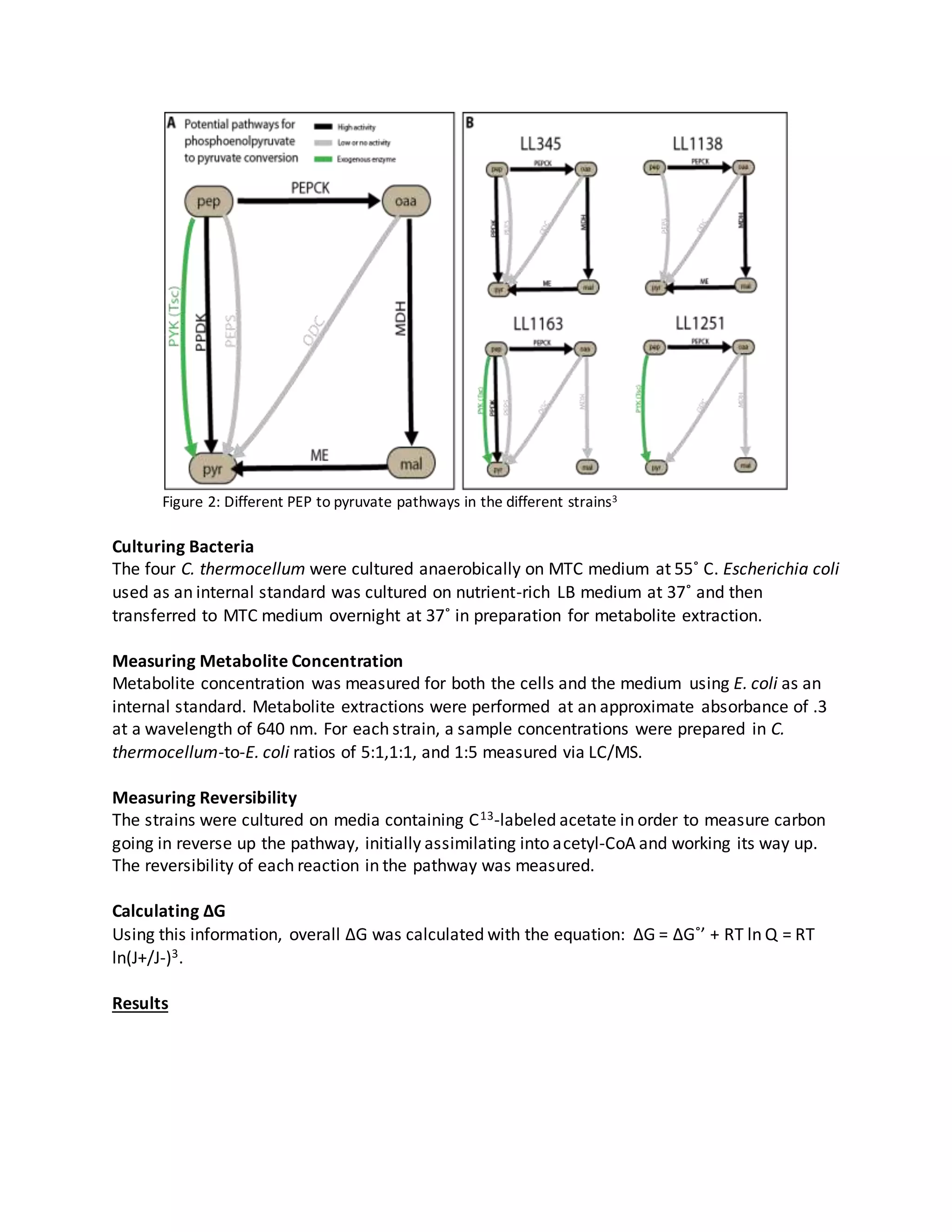
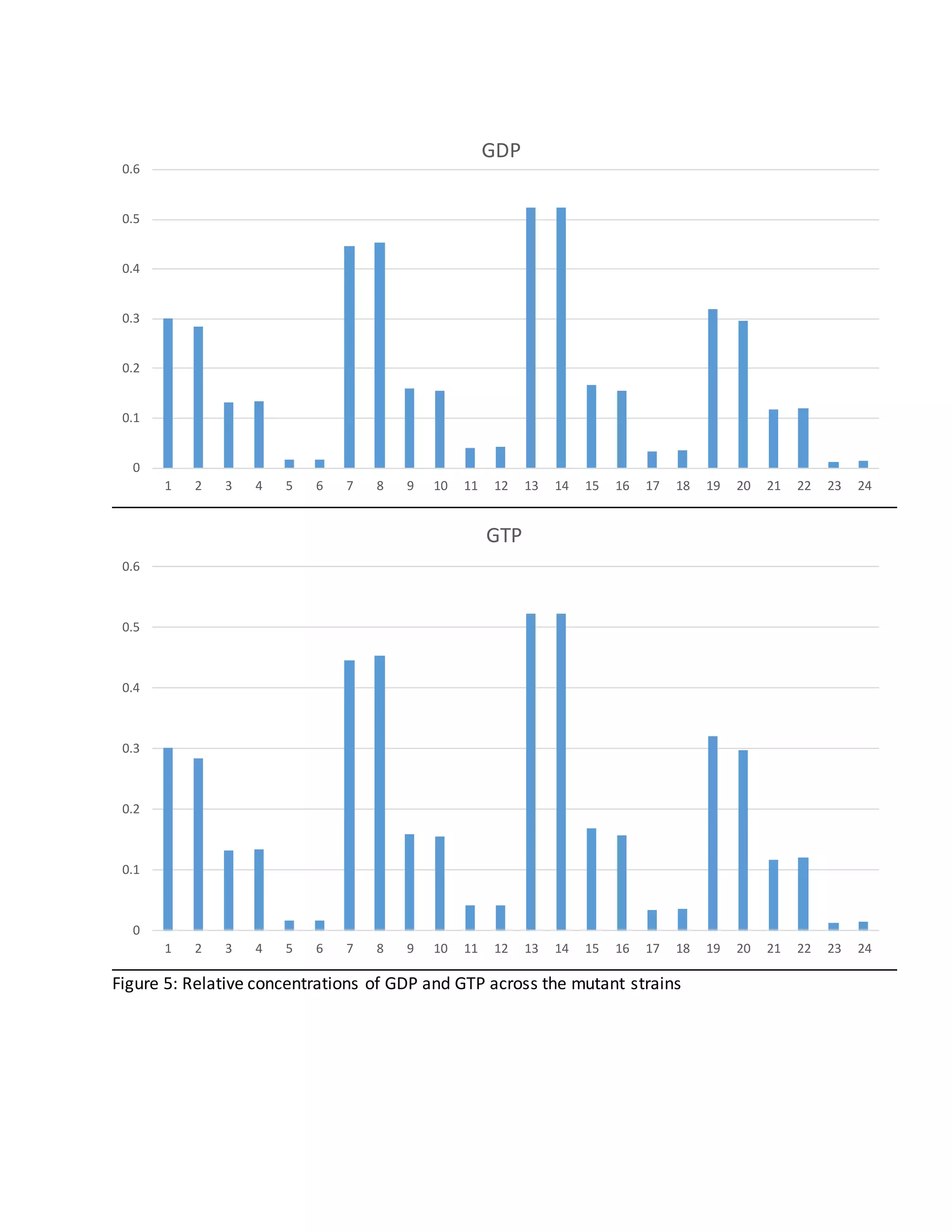
The document summarizes a study that analyzed 4 genetically engineered strains of Clostridium thermocellum with differing pathways for converting phosphoenolpyruvate (PEP) to pyruvate. The strains were cultured and their metabolite concentrations and reversibility of reactions were measured to calculate free energy changes. Strain 1138 diverted flux through the malate shunt, strain 1163 expressed an exogenous pyruvate kinase, and strain 1251 expressed pyruvate kinase and deleted PPDK and the malate shunt. Strain 1163 had higher GDP and GTP, suggesting its PEP to oxaloacetate conversion was more efficient. Further optimizing the PEP to pyruvate pathway, such as
![Metabolomic and thermodynamic analysis of C. thermocellum strains
engineered for high ethanol production
Jordan Brown1, Dave Stevenson2, Daniel Amador-Noguez2
1-Department of Botany-Microbiology (Genetics), Ohio Wesleyan University
2-Department of Bacteriology, University of Wisconsin-Madison
Introduction
Ethanol is a carbon neutral fuel that can be produced from the microbial conversion of
cellulosic biomass [1]. One microorganism that is being engineered to perform this conversion
is the aerobic thermophillic bacteria, Clostridium thermocellum. Past efforts that focused solely
on metabolomic analyses have been able to increase ethanol production, but only up to a
certain amount. Studies show that the overall pathway of this conversion is not as energetically
favorable as in other biofuel producing strains. This causes the conversion of cellulose to
ethanol to proceed slowly and, in some cases, allows for reactions in the pathway to proceed in
reverse. By making the change in free energy (∆G) more negative at key steps in the reaction,
the overall reaction will become more spontaneous and proceed at a faster rate. This would
increase ethanol production, and drive down the price, allowing ethanol-based biofuels to
better compete with fossil fuels at the pump.
One key step in the pathway to be optimized is the conversion of phosphoenolpyruvate (PEP) to
pyruvate. C. thermocellum utilizes the Emben-Meyerhof-Parnas (EMP) pathway, but unlike
other bacteria that use this pathway it does not have the enzyme pyruvate kinase (PYK), which
catalyzes the direct conversion of PEP to pyruvate. Instead it utilizes two different pathways.
One is the direct conversion of PEP to pyruvate by a different enzyme, pyruvate phosphate
dikinase (PPDK). This pathway has been experimentally shown not to be able to be the sole
pathway in PEP to pyruvate conversion. The other pathway is the malate shunt, which uses PEP
carboxylase (PEPCK), malate dihydrogenase (MDH) and malic enzyme (ME)1. This pathway has
been shown to be able to be the sole PEP to pyruvate pathway.
4 genetically engineered strains of C. thermocellum with differing pathways for the PEP to
pyruvate conversion were created and analyzed from an integrated metabolomic and
thermodynamic point of view. Comparison of alternate pathways for this conversion will allow
for determining the optimal PEP to pyruvate pathway to be used in C. thermocellum in
converting cellulosic biomass to ethanol.](https://image.slidesharecdn.com/18f65bde-10fc-4e37-8fb5-08825d007067-161025102119/75/JB-REU-Report-1-2048.jpg)
![Discussion
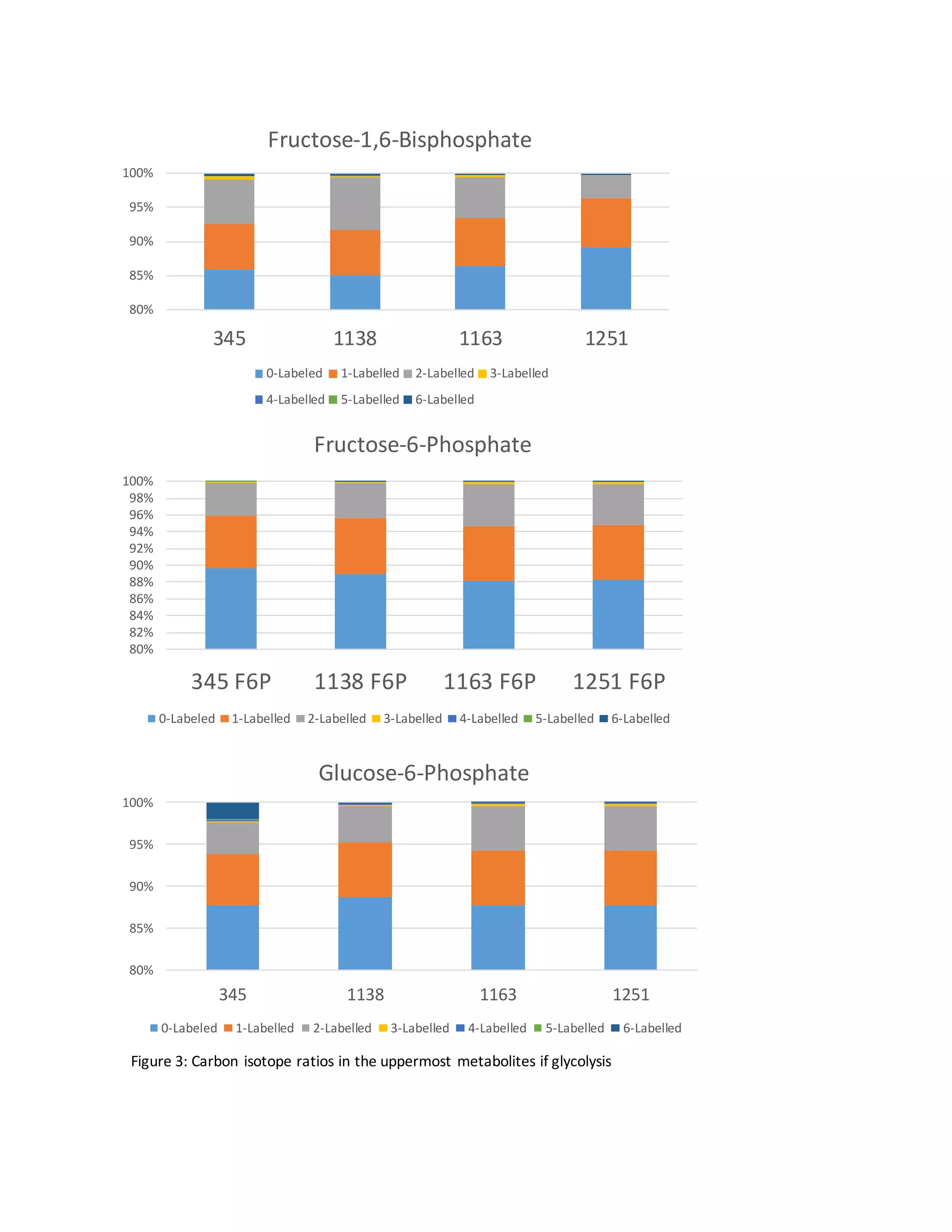
Glycolytic Reversibility
Contrary to most organisms, in which the reactions of upper glycolysis display essentially 100%
forward flux, the reactions in the EMP pathway of C. thermocellum display some extent of
reverse flux. This is particularly unique in the uppermost reactions of glycolysis[3].
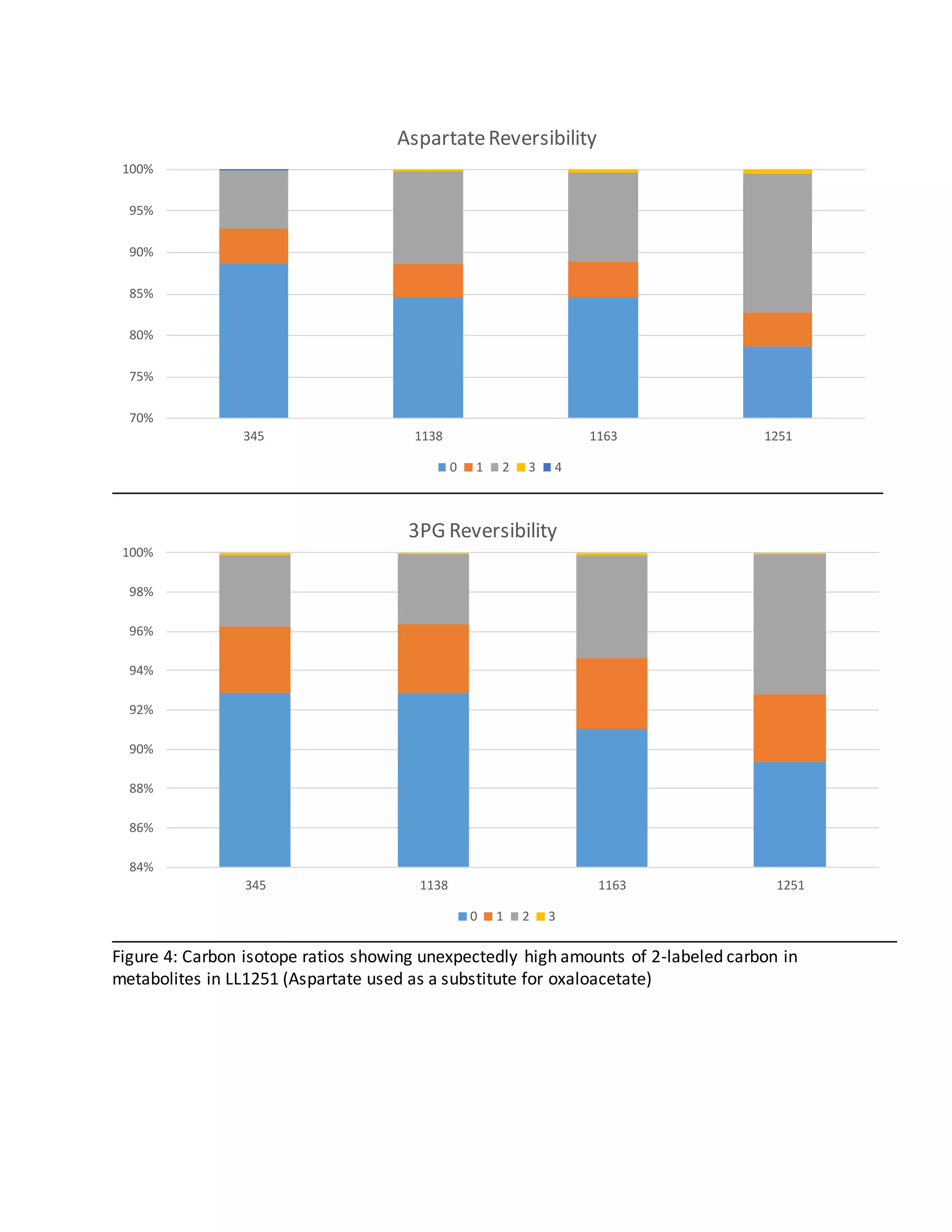
Secret Pathways
In LL1251 the only way for labeled carbon to make its way back through the pathway to
oxaloacetate is in reverse from pyruvate to PEP and then forward to oxaloacetate. Because the
enzyme responsible for PEP-> pyruvate (PYK) in LL1251 is relatively less reversible than those
used in other strains (PPDK or PYK and PPDK together), it would be expected for less label to be
found in oxaloacetate in LL1251. This however is not the case [4]. This suggests that there are
additional pathways present in this mutant involving these metabolites that have yet to be
elucidated.
Increase GDP and GTP in LL1163
Compared to the other strains, there is a considerably higher relative concentration of GDP and
GTP in LL1163. This is particularly of interest because GTP production is linked to conversion of
PEP to oxaloacetate.
Further Research
C. thermocellum contains the gene for oxaloacetate decarboxylase (ODC), an enzyme that
catalyzes the conversion of oxaloacetate to pyruvate. However, there is no ODC expression in
wild type C.thermocellum. It would be of use to investigate this, and attempt to express this
gene to create a novel and potentially more efficient pathway for the PEP to pyruvate
conversion.
References
1) Olsen,Daniel G.,Manuel Hörl,TobiasFuhrer,JingxuanCui,MarybethI.Mahoney,Daniel Amador-
Noguez,LiangTian,Uwe Sauer,and Lee R. Lynd."Conversionof PhosphorenolpyruvatetoPyruvate in
ClostridiumThermocellum." *Currently underReview and NotYet Published (n.d.):n.pag.Print.
2) Chinn,Mari, andVeronicaMbaneme."ConsolidatedBioprocessingforBiofuel Production:Recent
Advances."EECTEnergy and Emission ControlTechnologies (2015): 23. Web.
3) Park, JunyoungO.,SaraA. Rubin,Yi-FanXu,Daniel Amador-Noguez,JingFan,TomerShlomi,and
JoshuaD. Rabinowitz."MetaboliteConcentrations,FluxesandFree EnergiesImplyEfficientEnzyme
Usage."NatureChemical Biology NatChemBiol (2016): n.pag. Print.
4) Flamholz,A.,E.Noor,A. Bar-Even,W.Liebermeister,andR.Milo."GlycolyticStrategyasa Tradeoff
between EnergyYieldandProteinCost." Proceedingsof theNationalAcademy of Sciences 110.24
(2013): 10039-0044. Print.](https://image.slidesharecdn.com/18f65bde-10fc-4e37-8fb5-08825d007067-161025102119/75/JB-REU-Report-7-2048.jpg)