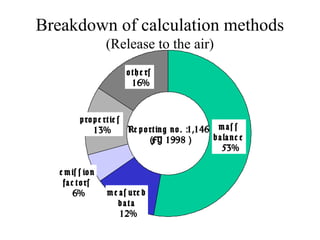
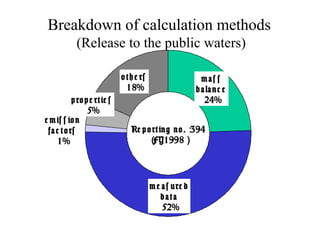
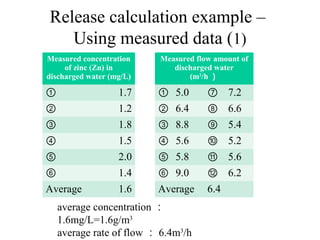
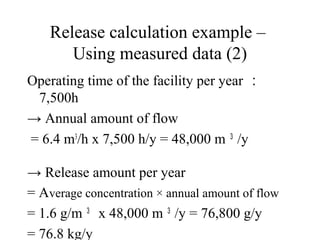
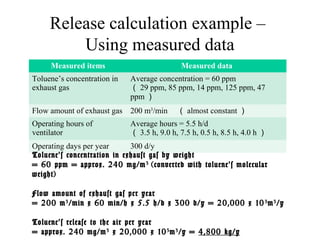
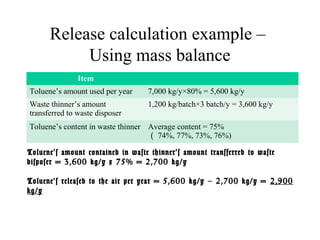
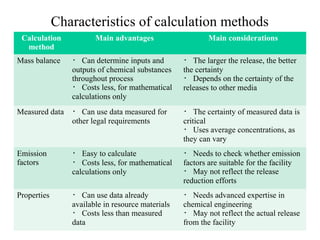
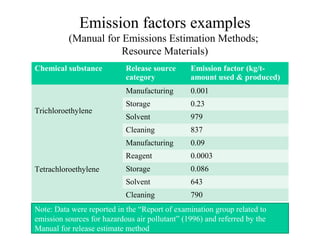
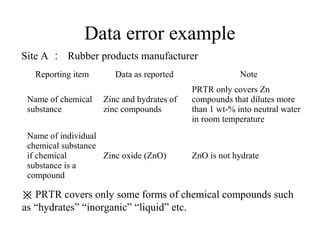
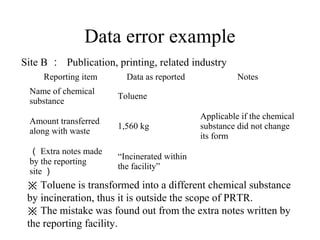
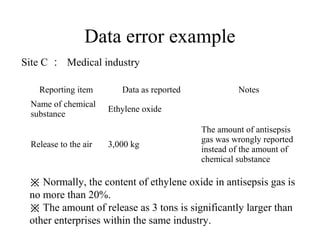
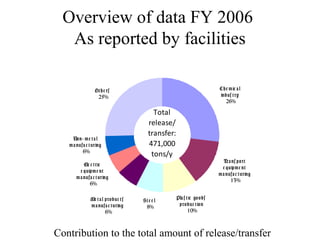
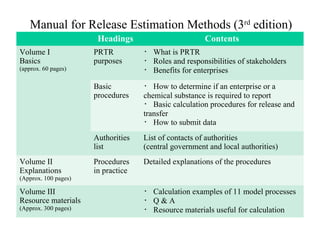
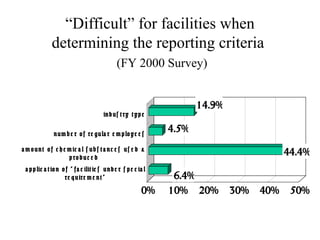
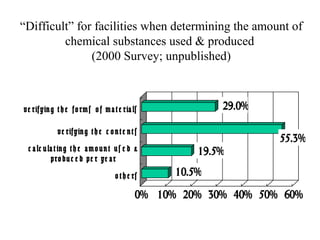
The document summarizes data reporting by enterprises under Japan's PRTR (Pollutant Release and Transfer Register) system. It provides an overview of reported releases by industry in 2006, with the chemical industry contributing 26% of the total amount. It also outlines the PRTR manual's contents to guide enterprises through the reporting process, including determining reporting criteria and calculating release amounts. Challenges for enterprises include accurately verifying chemical contents and amounts used, as well as selecting the proper calculation method from options like mass balance, measured data, and emission factors.




![MSDS System
[MSDS System]
MSDS (Material Safety Data Sheet) is required for
an enterprise when it gives/provides a chemical
substance covered by PRTR or a product
containing such chemical substance to other
enterprises
[Scope of enterprises subject to MSDS System]
All enterprises which use/produce the chemical
substances covered by PRTR](https://image.slidesharecdn.com/japan2-datareportingbyenterprises08-12-08-130608042025-phpapp01/85/Japan-2-data-reporting-by-enterprises08-12-08-9-320.jpg)