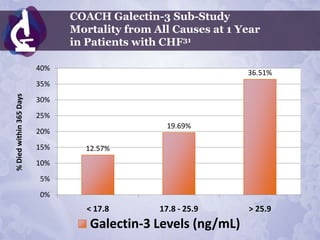
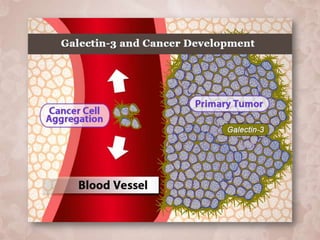
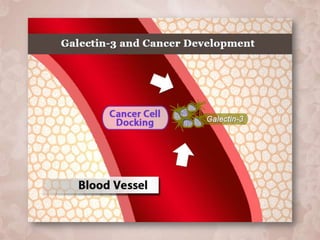
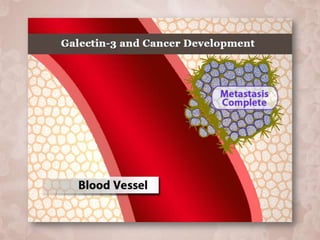
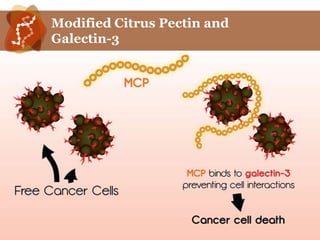
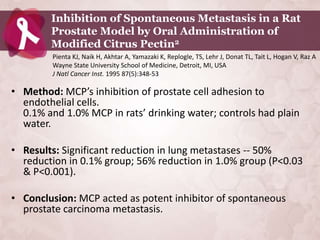
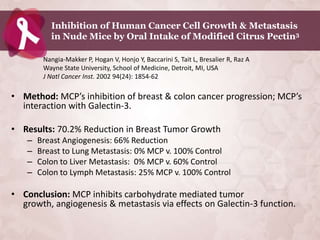
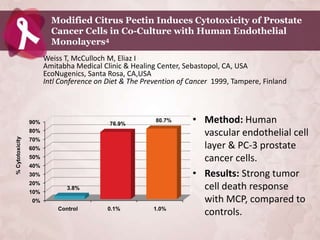
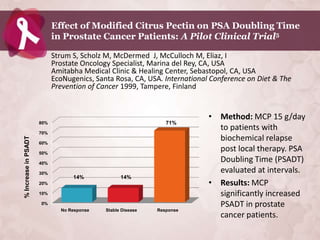
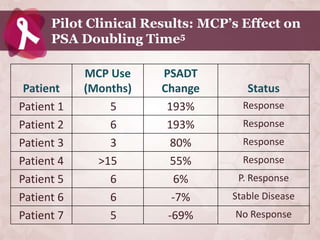
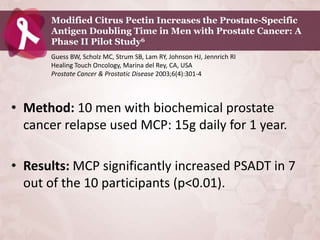
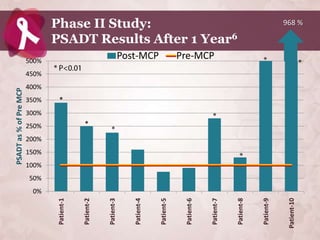
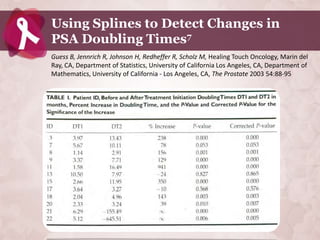
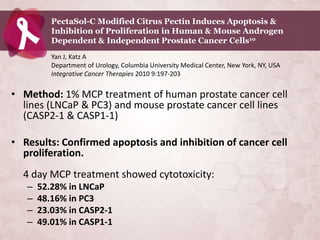
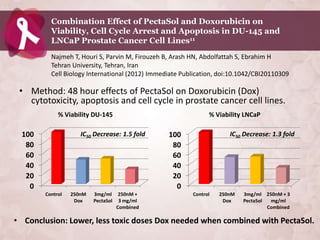
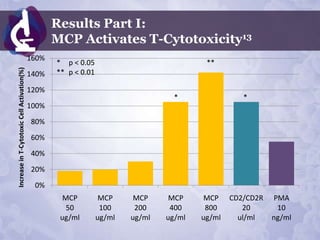
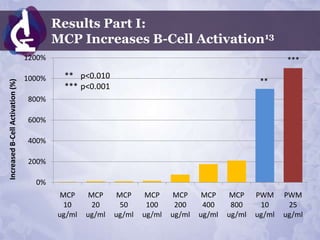
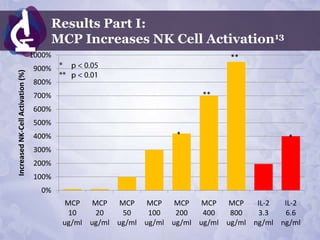
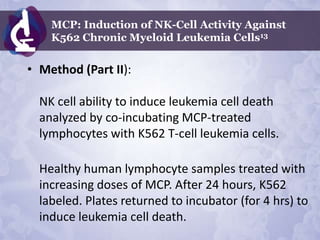
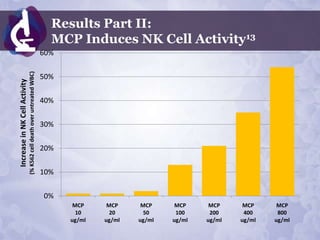
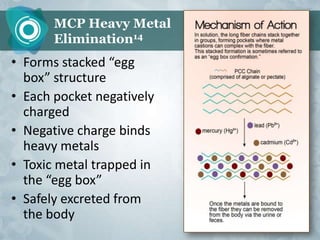
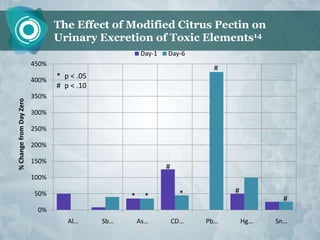
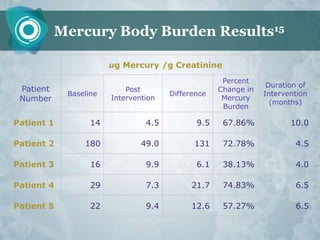
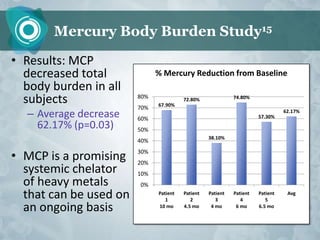
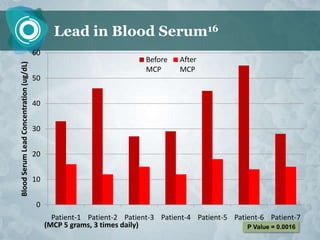
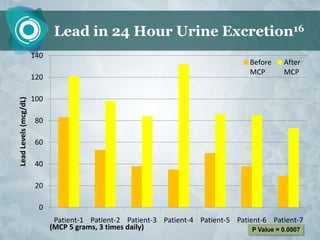
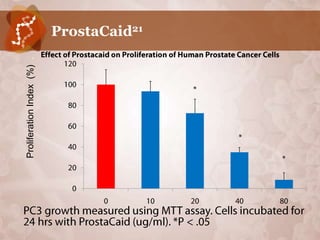
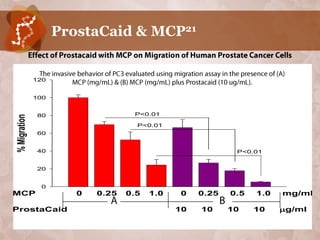
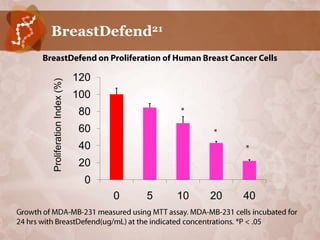
Galectin-3 plays an important regulatory role in cancer, inflammation, and fibrosis. Modified citrus pectin (MCP) binds to and blocks galectin-3, inhibiting cancer cell aggregation and metastasis. Several studies show MCP reduces tumor growth, angiogenesis, and metastasis in animal models of prostate, breast, and colon cancer. Clinical trials demonstrate MCP increases prostate-specific antigen doubling time in prostate cancer patients and provides clinical benefits such as improved quality of life for patients with advanced cancers. MCP may also enhance the effects of chemotherapy drugs and protect against post-radiation damage. Research indicates MCP activates T-cytotoxic cells, B-cells, and natural killer cells, stimulating anti-cancer








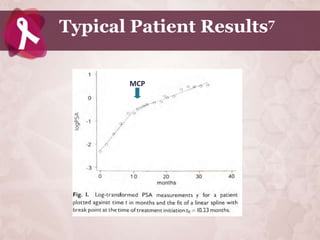












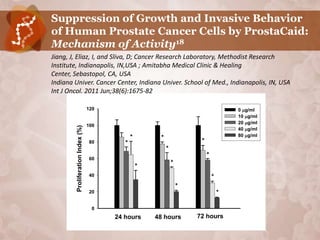
![Effect of ProstaCaid on Invasive Behavior
of Prostate Cancer Cells18
120 120
Cell migration [%]
Cell adhesion [%]
100 100
80 * 80 *
60 60
* *
40 40
20 20
0 0
0 20 40 80 0 20 40 80
ProstaCaid (g/ml) ProstaCaid [g/ml]
120
Cell invasion [%]
100
*
80
*
60
40
20
0
0 20 40 80
ProstaCaid [g/ml]](https://image.slidesharecdn.com/isaaceliazoncanpfeb2012phoenix-120210130833-phpapp01/85/Isaac-Eliaz-OncANP-Feb-2012-58-320.jpg)


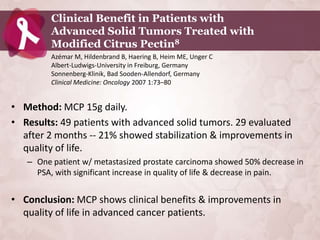
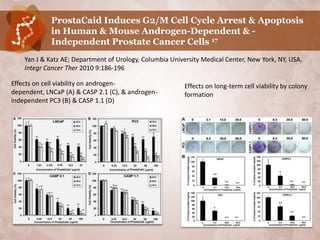
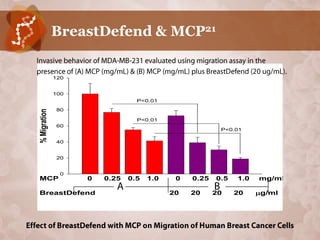
![Suppression of Proliferation & Invasive
Behavior of Human Metastatic Breast Cancer
Cells by Dietary Supplement BreastDefend20
Jiang J, Wojnowski R, Jedinak A, Sliva D; Cancer Research Laboratory, Methodist Research
Institute, Indianapolis, IN, USA Department of Medicine, Indiana University. Cancer
Center, School of Med., Indiana University, Indianapolis, IN, USA; Integr Cancer Ther.
2011; Jun 10 (2):192 – 200
0g/ml
120 10 g/ml
20 g/ml
30 g/ml
100
40 g/ml
Proliferation Index [%]
80
* *
60
* *
*
40
*
20
* * * *
0
24 hours 48 hours 72 hours](https://image.slidesharecdn.com/isaaceliazoncanpfeb2012phoenix-120210130833-phpapp01/85/Isaac-Eliaz-OncANP-Feb-2012-64-320.jpg)









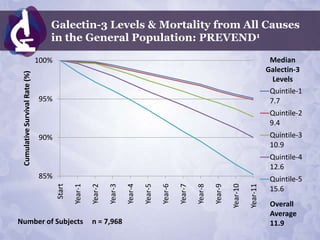
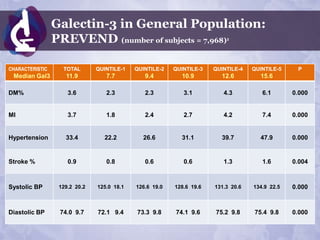
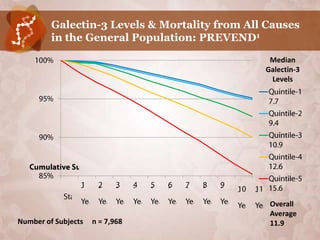
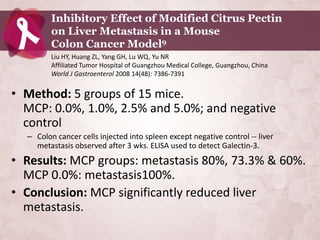
![Galectin-3 in General Population:
PREVEND (number of subjects = 7,968) 1
CHARACTERISTIC TOTAL QUINTILE-1 QUINTILE-2 QUINTILE-3 QUINTILE-4 QUINTILE-5 P
Median Gal3 11.9 7.7 9.4 10.9 12.6 15.6
1.29 0.89 1.04 1.33 1.53 1.98
CRP 0.000
[0.56-3.00] [0.39-2.16] [0.49-2.40] [0.58-2.92] [0.71-3.42] [0.85-4.28]
Cholesterol 5.66 1.12 5.41 1.05 5.56 1.10 5.68 1.11 5.79 1.11 5.91 1.17 0.000
LDL 3.69 1.05 3.47 1.00 3.60 1.01 3.71 1.04 3.77 1.05 3.90 1.06 0.000
1.27 1.32 1.28 1.25 1.24 1.24
HDL 0.000
[1.03+1.56] [1.07-1.62] [1.04-1.57] [1.03+1.55] [1.03-1.53] [0.99-1.52]
1.16 1.02 1.11 1.17 1.23 1.31
Triglycerides 0.000
[0.85-1.68] [0.75-1.43] [0.82-1.59] [0.86-1.69] [0.89-1.78] [0.95-1.92]](https://image.slidesharecdn.com/isaaceliazoncanpfeb2012phoenix-120210130833-phpapp01/85/Isaac-Eliaz-OncANP-Feb-2012-83-320.jpg)