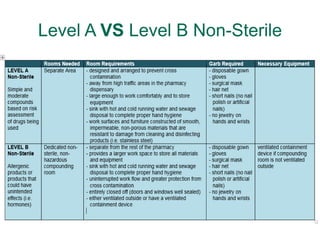
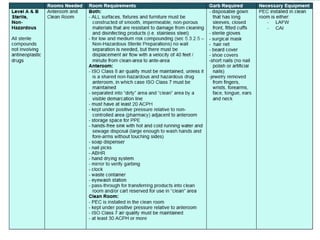
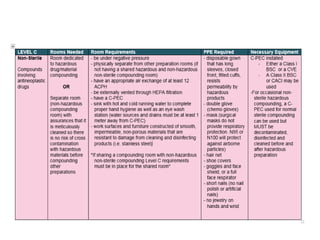
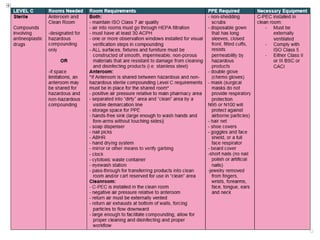
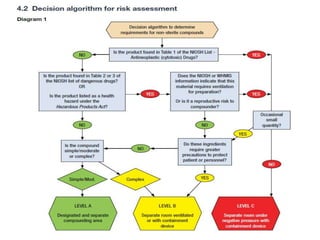
NAPRA has introduced three sets of Model Standards for pharmacy compounding in sterile and non-sterile preparations. In December 2017, NAPRA adopted all three Model Standards which classify compounding based on levels of hazard and risk. Level C is for hazardous sterile and non-sterile compounds using certain drugs. Levels A and B are for non-hazardous sterile compounds, while Level B is also for some non-sterile compounds involving allergenic or hormonal drugs. Level A includes many simple non-sterile preparations. A risk assessment considers factors like drug, dosage form, exposure risk and is needed for certain compounds.