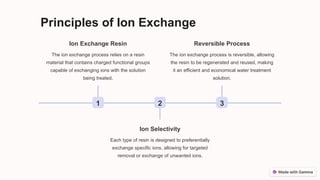
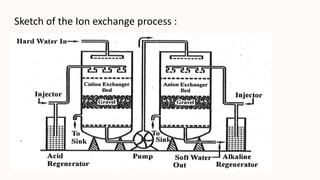
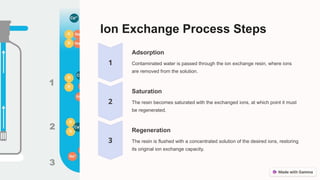
The ion exchange method is a water treatment technique that utilizes specialized resins to selectively remove ions from solutions, making it effective for water purification, chemical processing, metal recovery, and decontamination. The process involves the use of cation and anion exchange resins, which are capable of exchanging specific ions and can be regenerated for multiple uses. While efficient and cost-effective, the method has limitations such as resin fouling, the generation of waste during regeneration, and finite resin capacity.