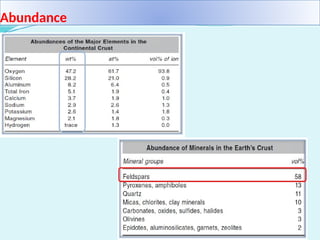
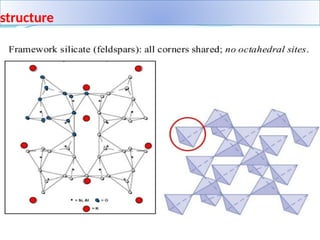
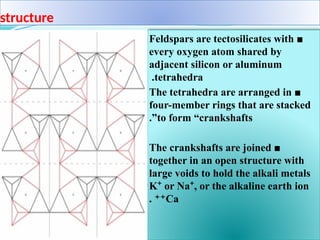
Feldspar is the most common rock-forming mineral, constituting about 60% of the Earth's crust and is present in various rock types including igneous, metamorphic, and sedimentary rocks. It is crucial in ceramics as a flux that enhances the strength and hardness of ceramic products by forming a glassy phase during firing. Feldspar is also widely used in the manufacturing of glass and various industrial applications due to its alumina and alkali content.