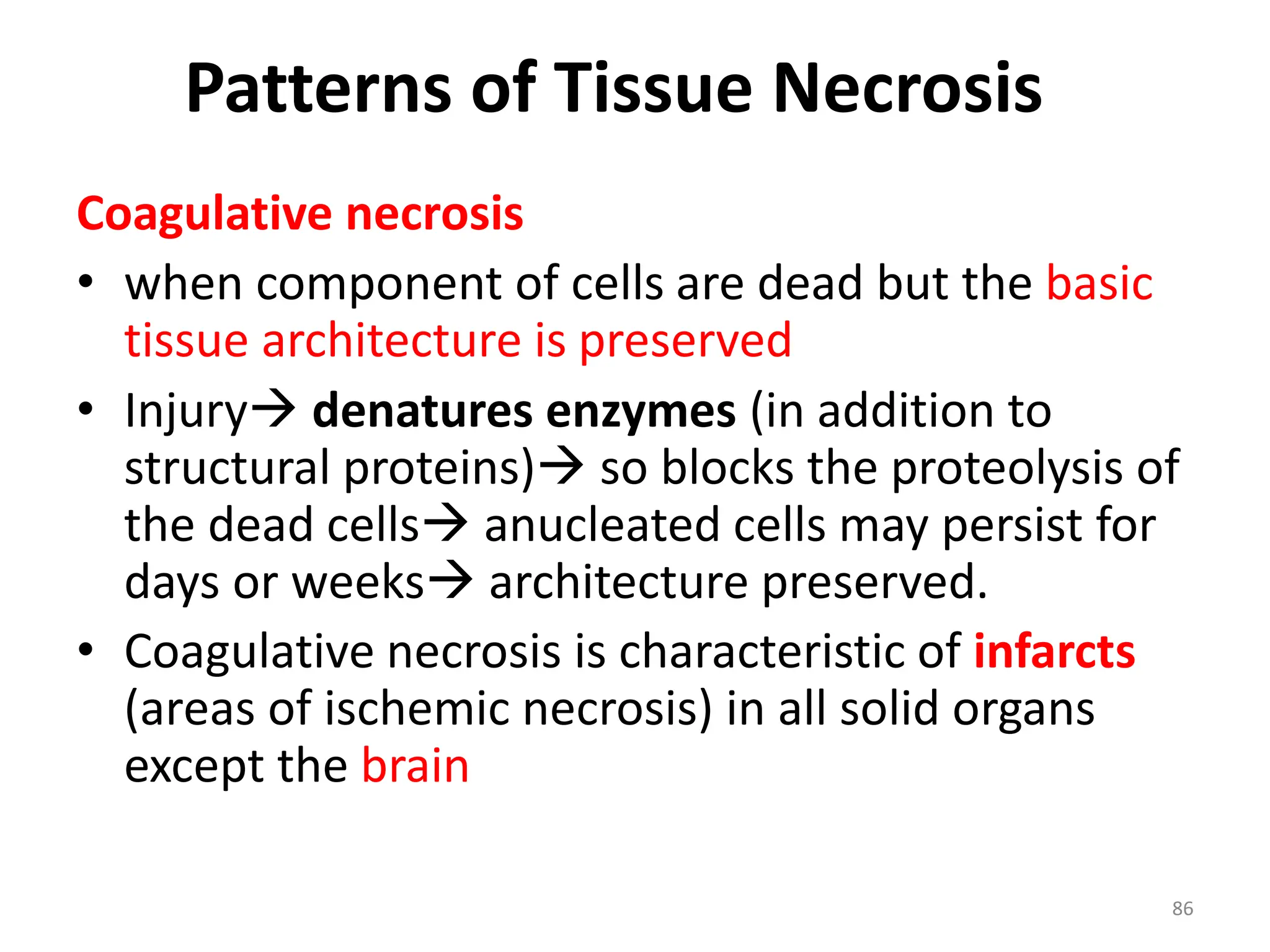
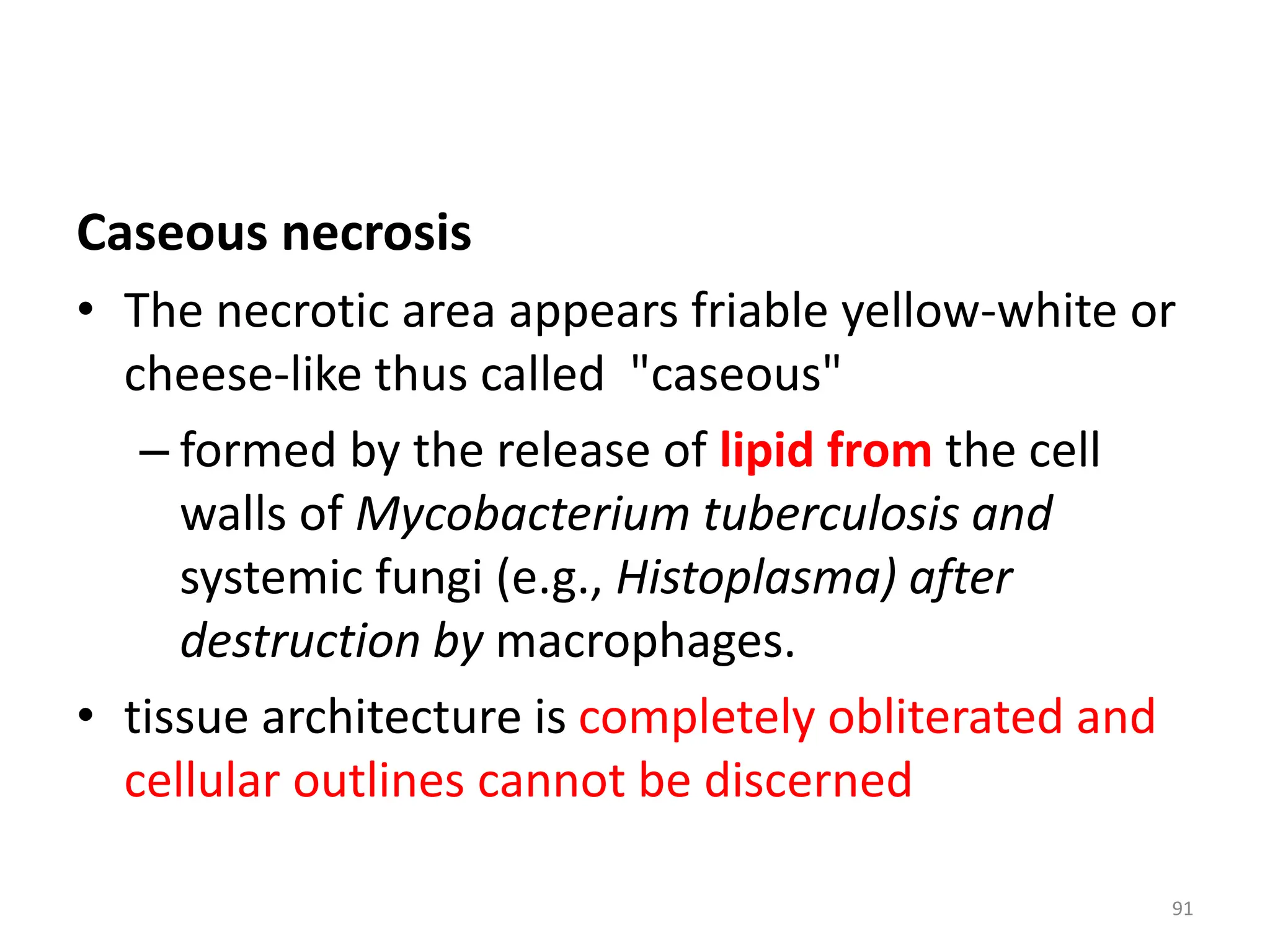
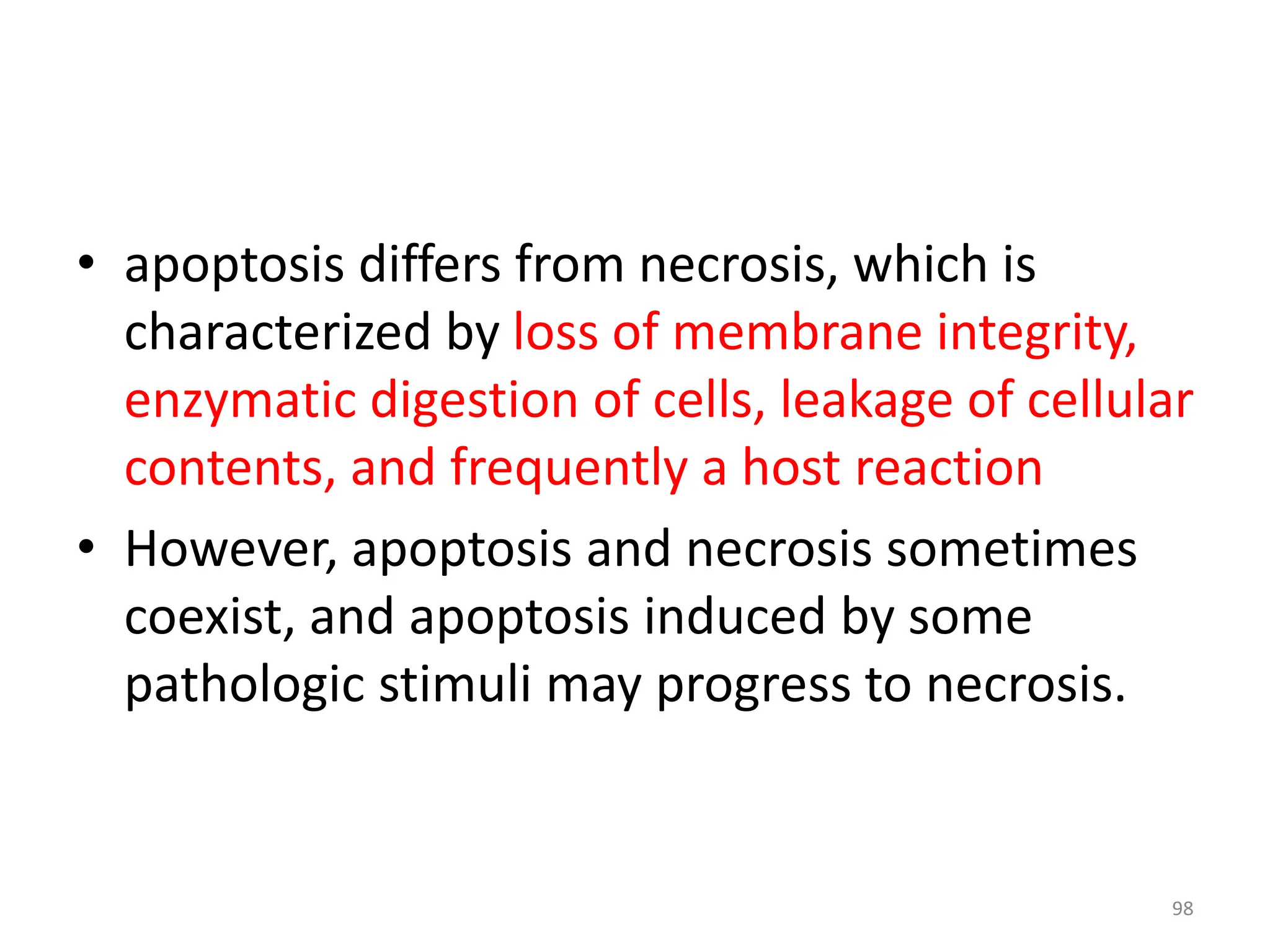
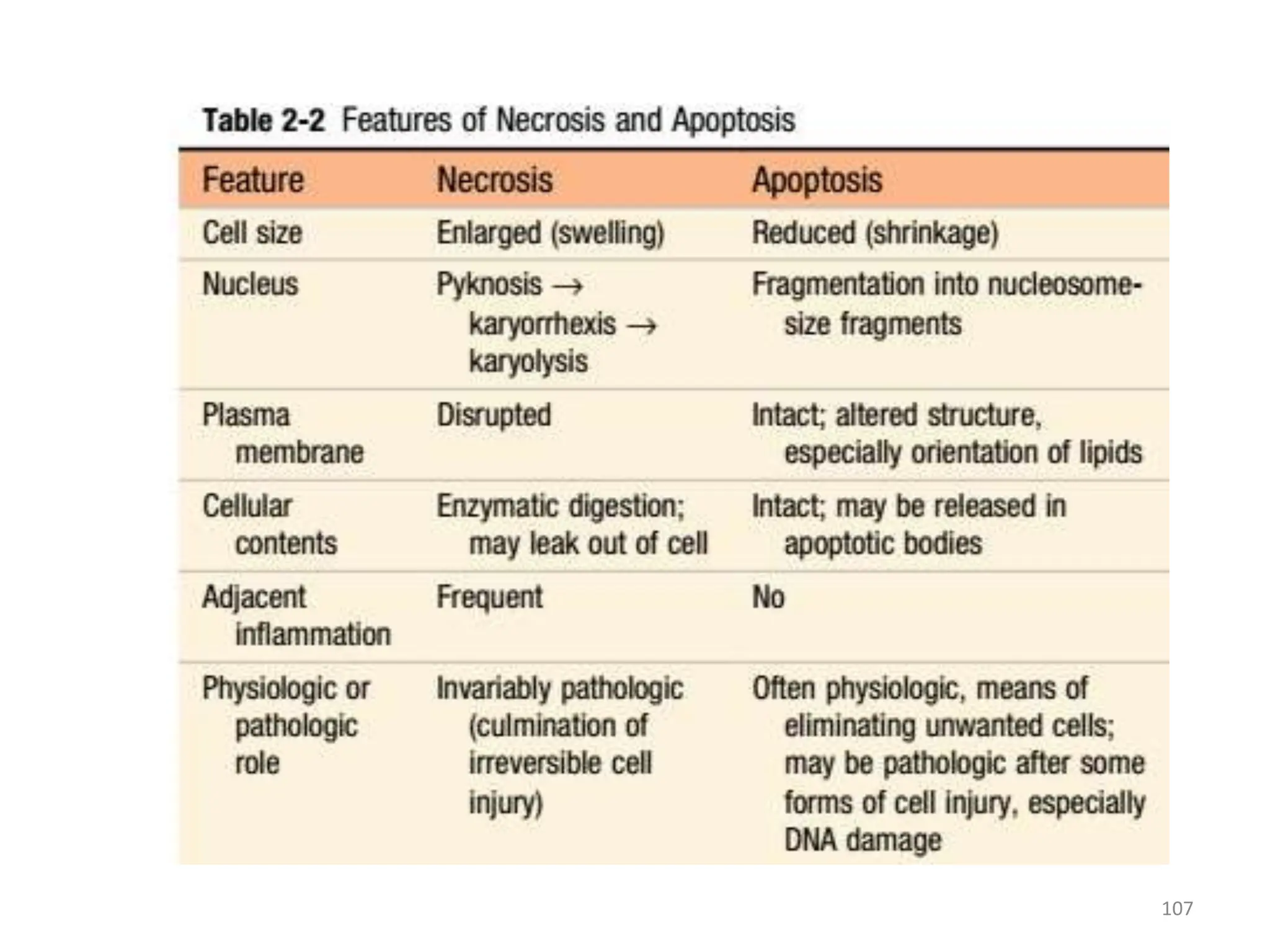
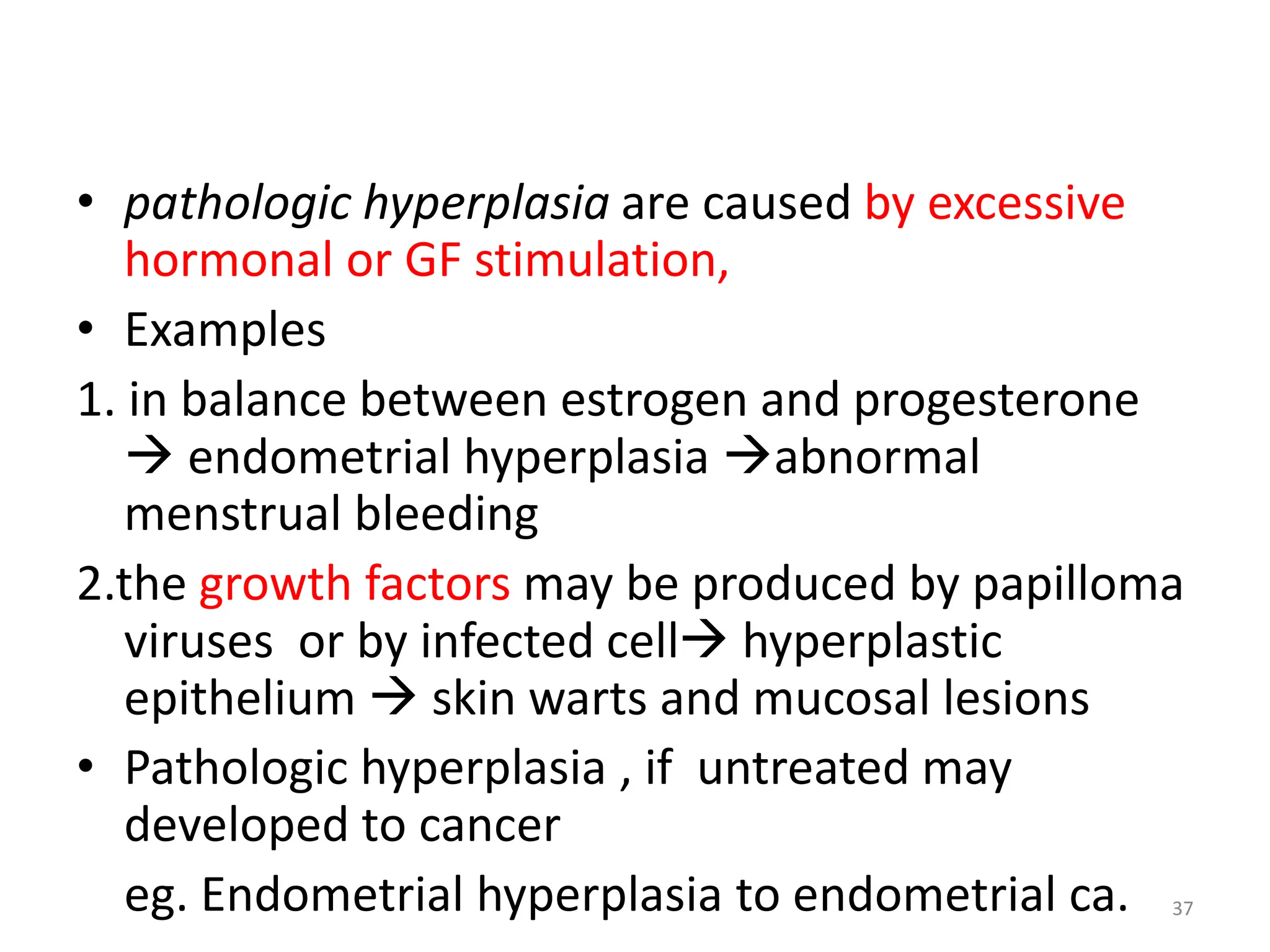
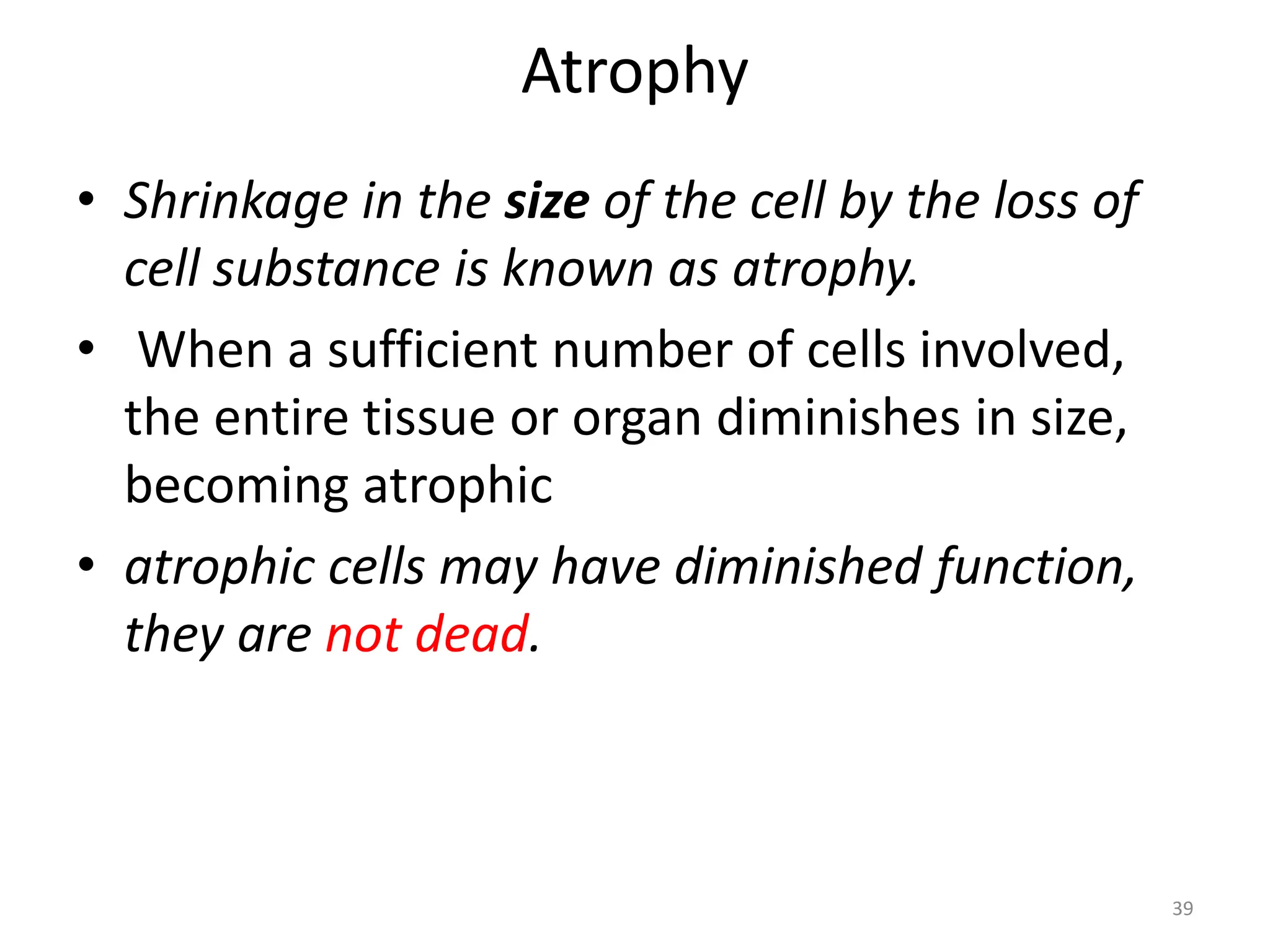
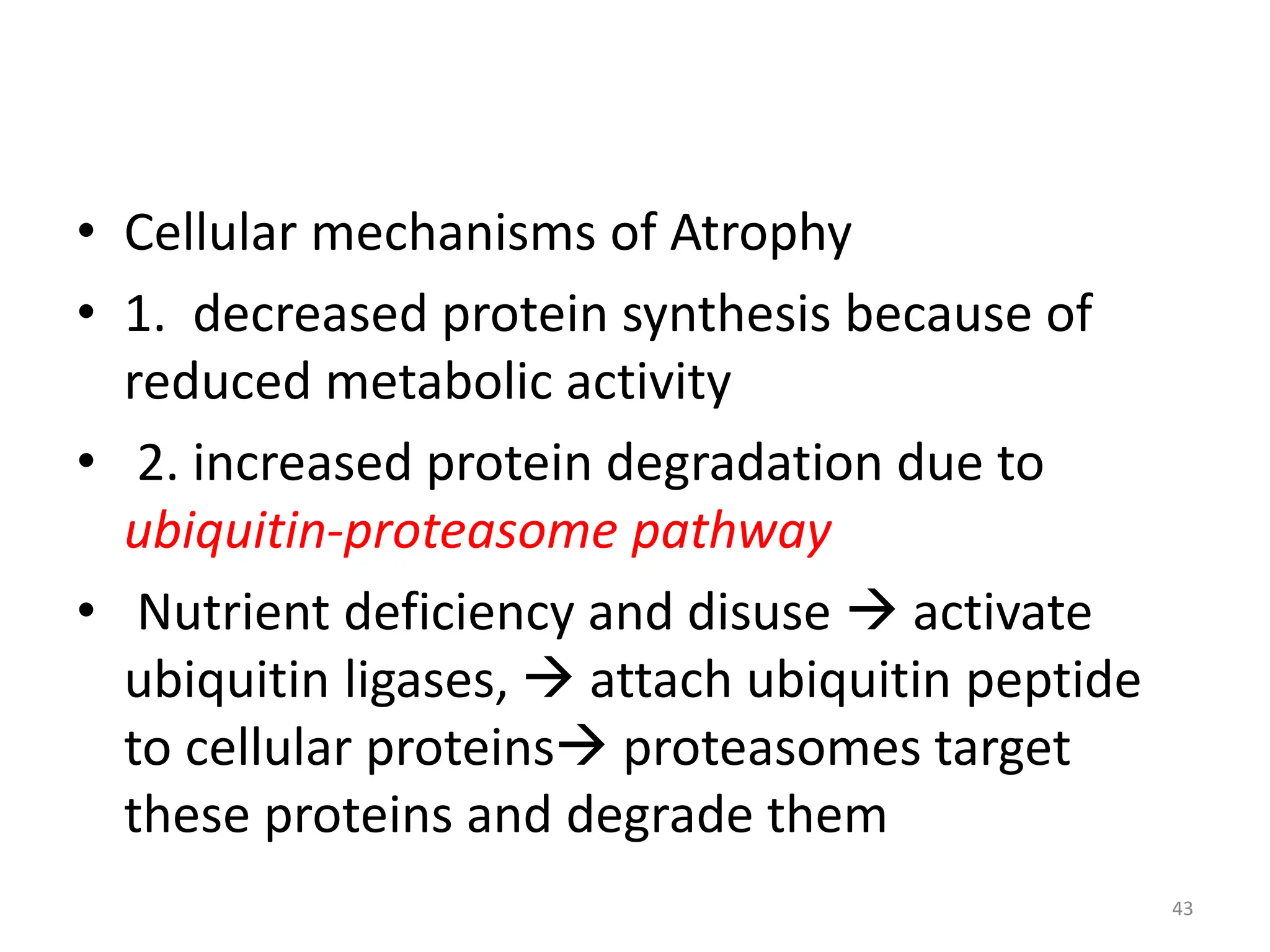
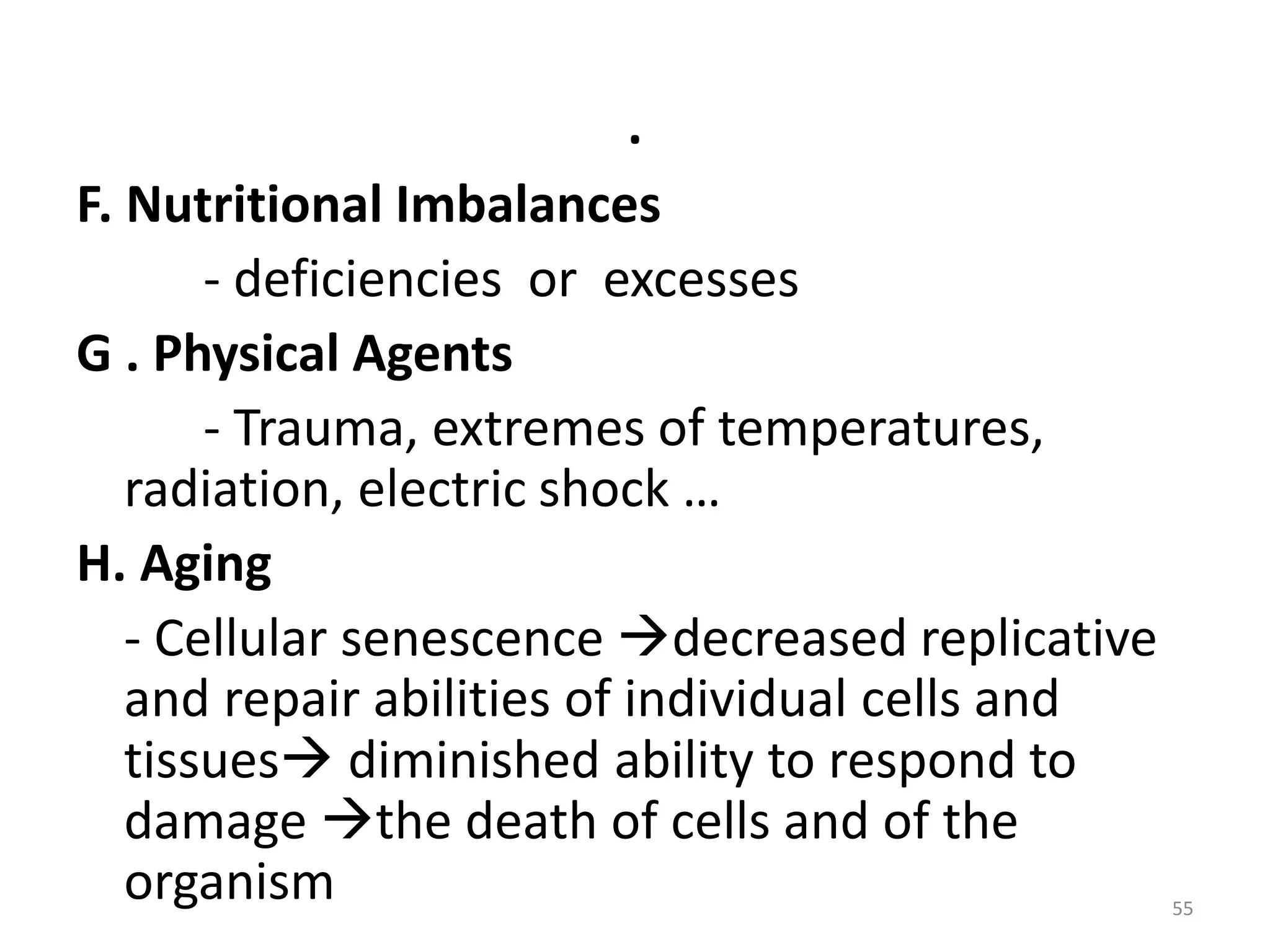
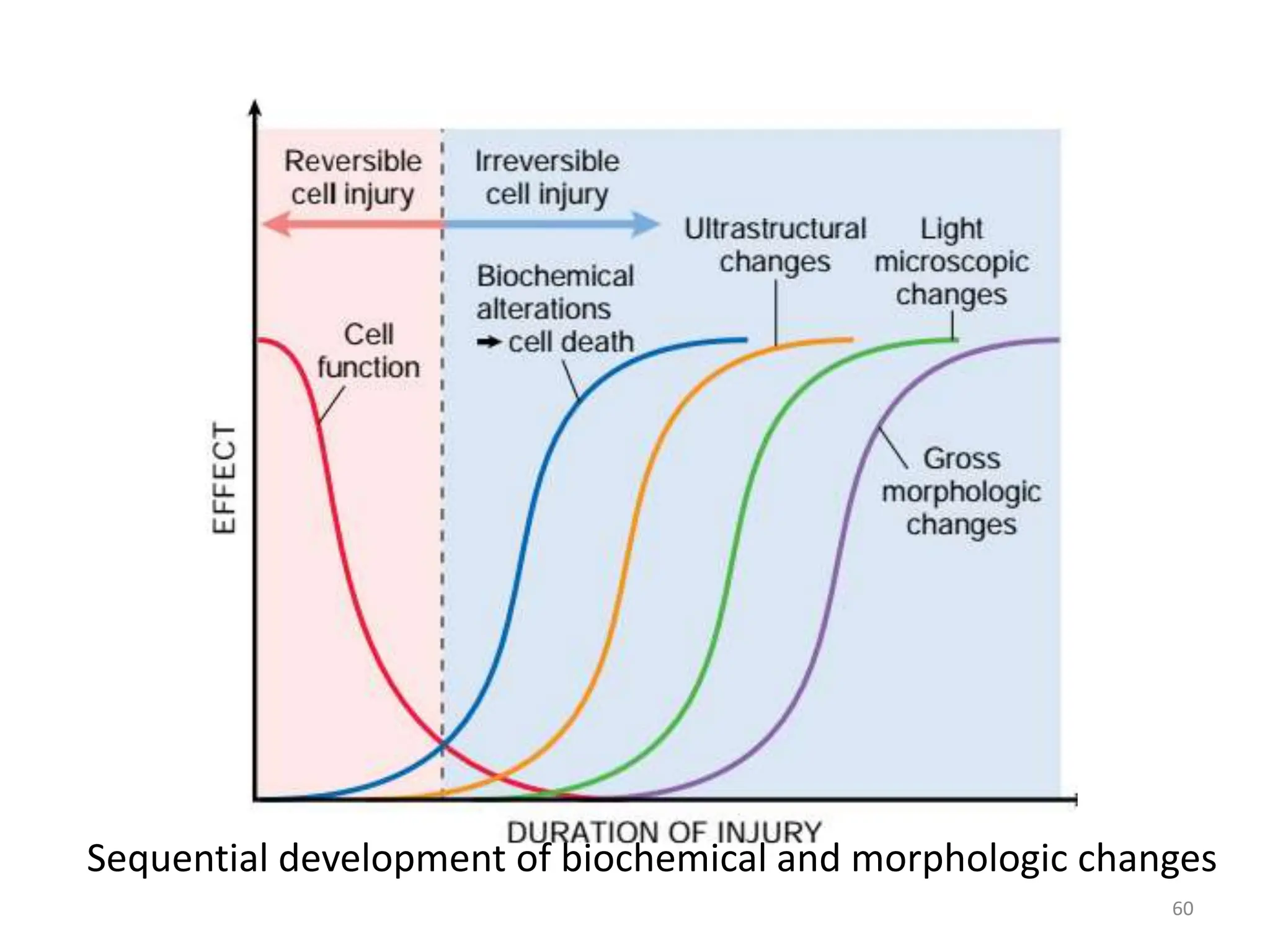
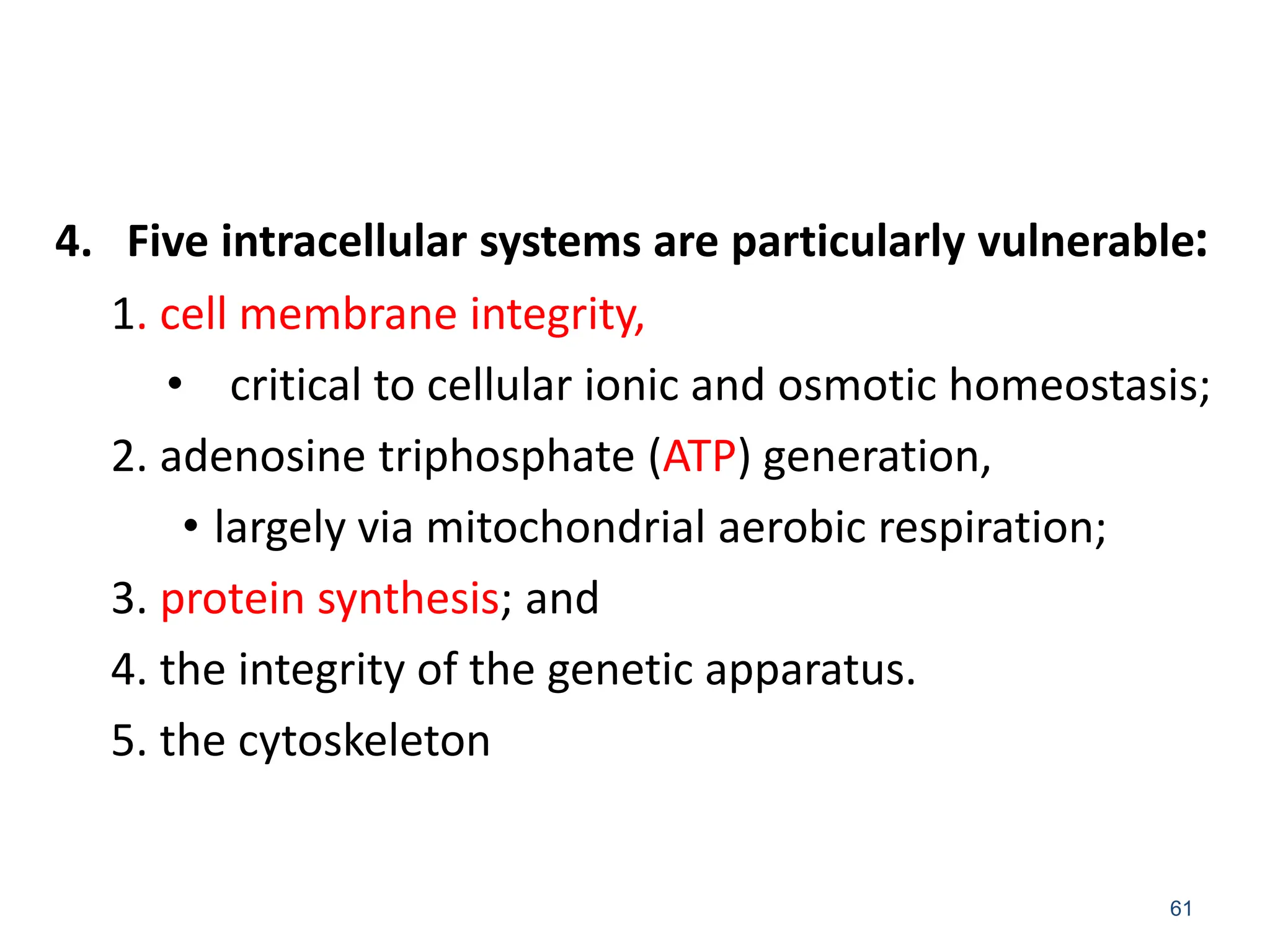
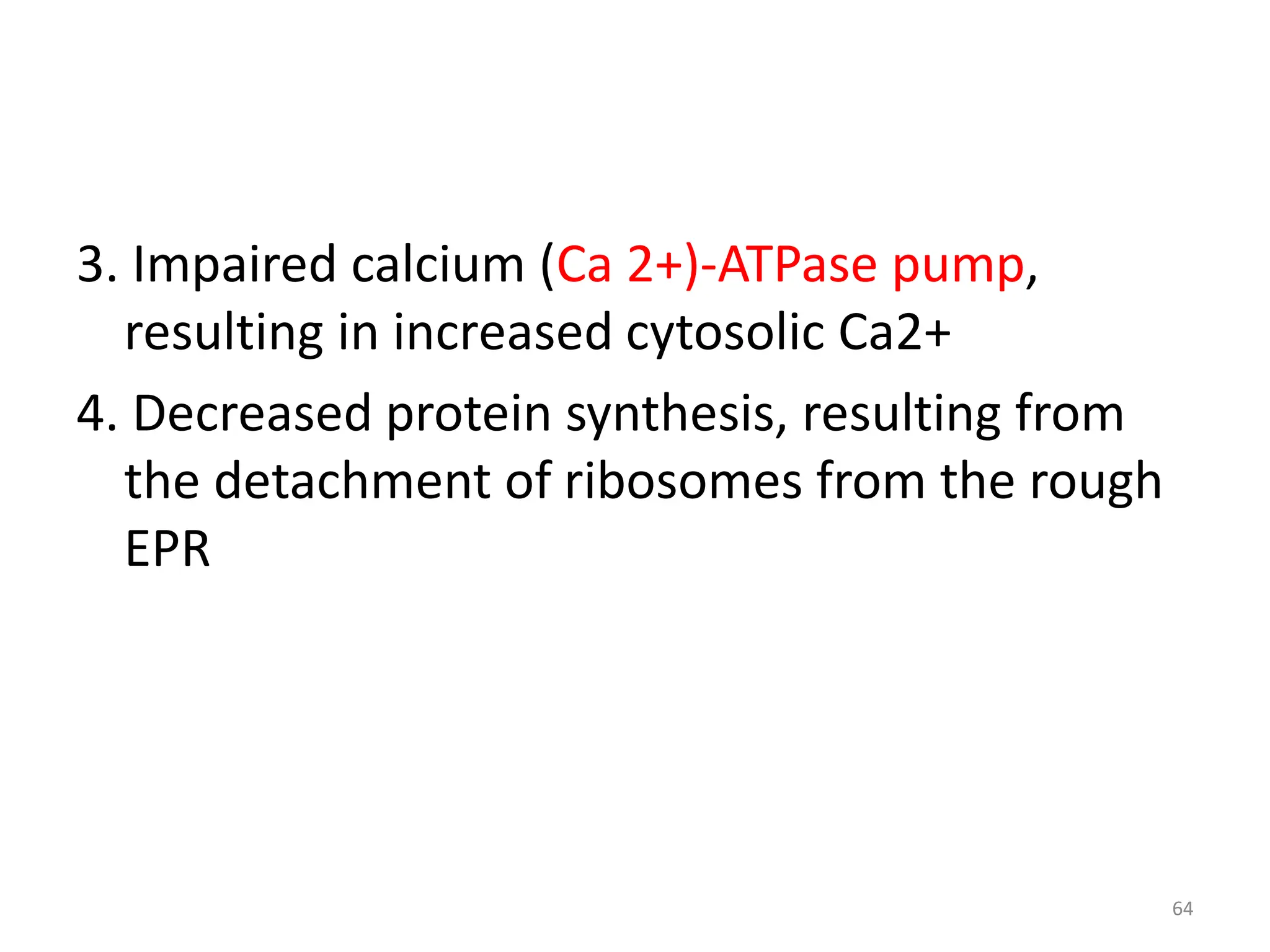
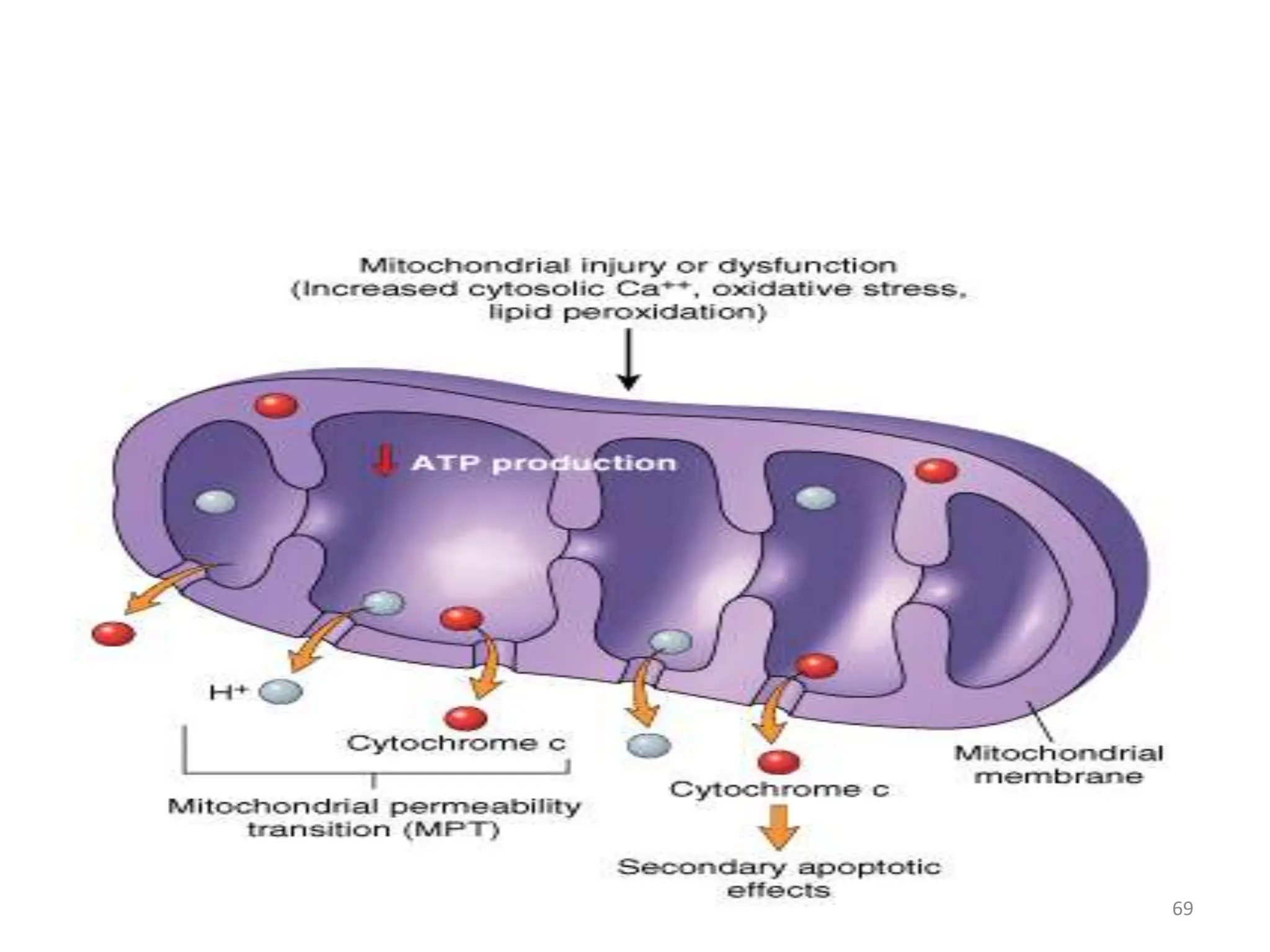
The document provides an overview of pathology, outlining its definition as the scientific study of disease and its division into general and systemic pathology. It details the four core aspects of disease (etiology, pathogenesis, morphologic changes, and functional derangements) and various diagnostic techniques used in pathology. Additionally, it discusses cellular responses to stress, including adaptations such as hypertrophy, hyperplasia, atrophy, and metaplasia, along with causes of cell injury and the mechanisms leading to irreversible damage and cell death.




































































![Nuclear changes: - due to
nonspecific DNA breakdown
• Pyknosis = nuclear
condensation(shrinkage) and
↑ basophilia
• Karyorrhexis [rhexis,
rupture]= nuclear
fragmentation
• Karyolysis= loss of DNA → ↓
basophilia
85](https://image.slidesharecdn.com/introductionandcellinjury-240420125654-6305888e/75/introduction-and-cell-injury-MATERIAL-pptxAnatomy-85-2048.jpg)