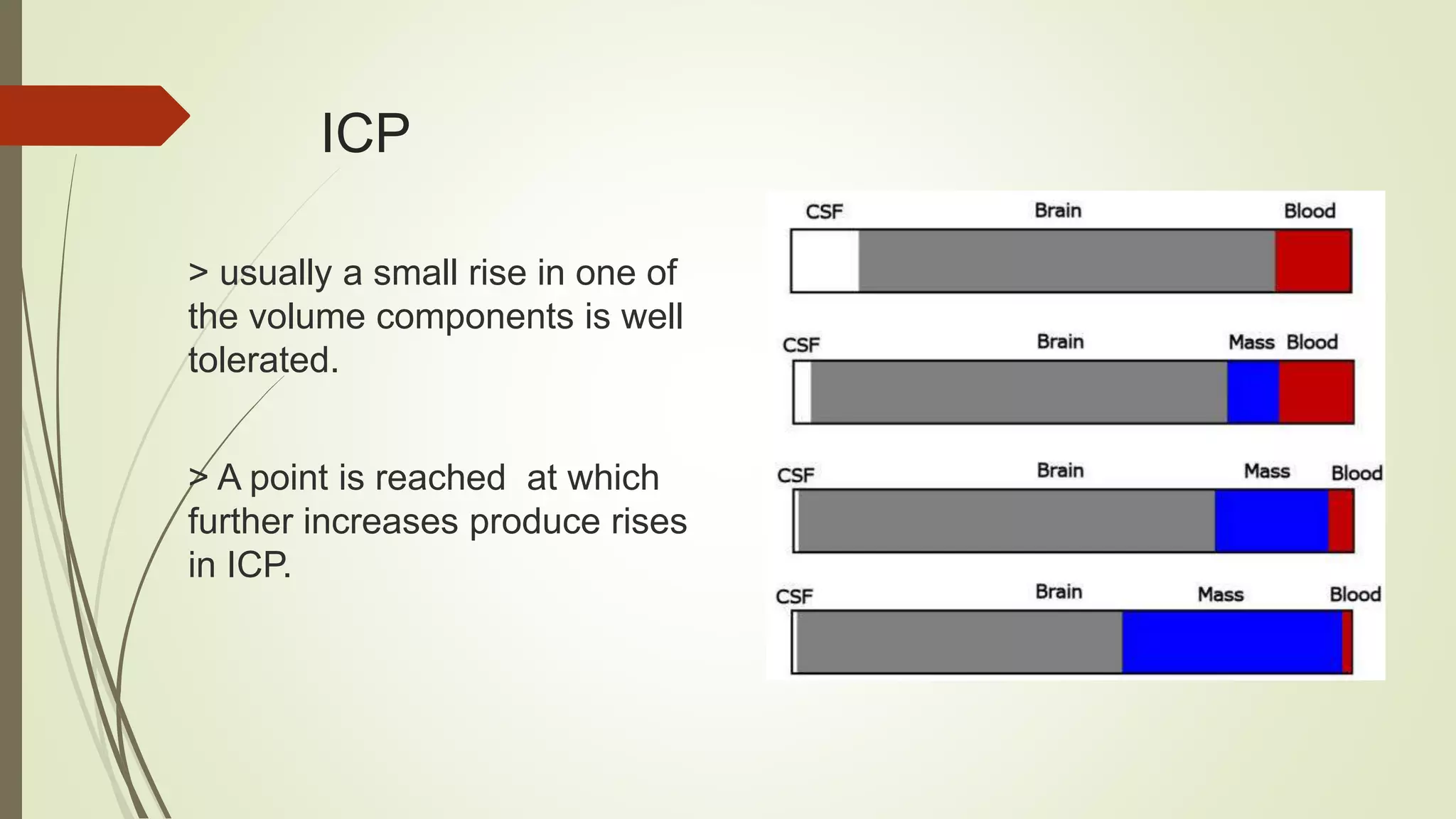
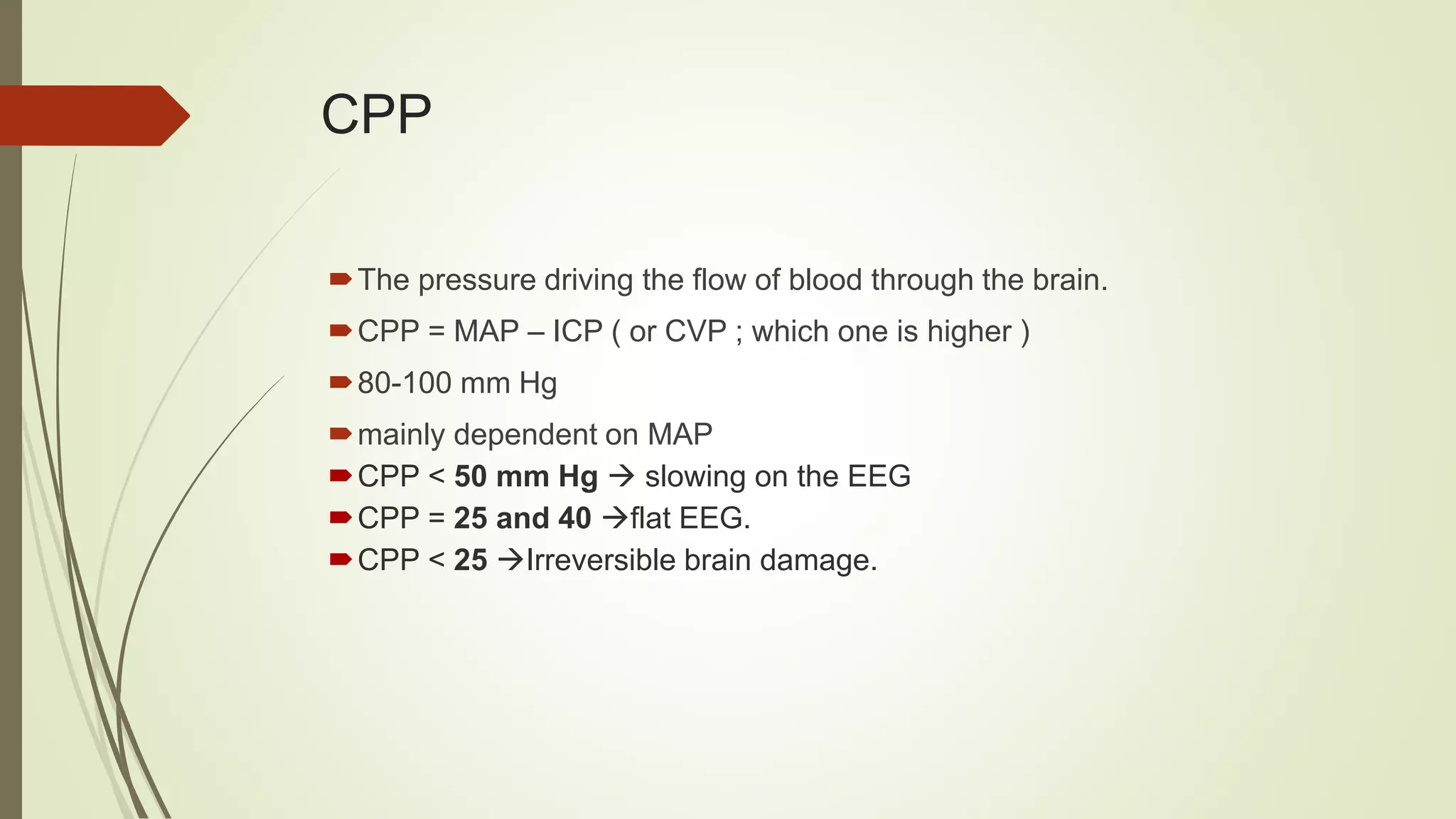
This document discusses various topics related to intracranial hemorrhage including terminology, intracerebral hemorrhage, intracranial pressure, cerebral blood flow, cerebral perfusion pressure, management goals, preventing hematoma expansion through blood pressure control and anticoagulant reversal, intracranial pressure management, surgery considerations, medical care, post-stroke seizures, and reperfusion therapy for ischemic stroke.











































![Hemodynamic and fluid management
Approximately 80 % of AIS patients are hypertensive [ SBP>140 mmHg] at
presentation.
Severe hypertension likely contributes to cardiorespiratory complications and
promotes cytotoxic edema and hemorrhagic transformation
There is a U-shaped relationship between BP and outcome after AIS, with both
high and low BP having adverse effects on outcome
Although high BP is independently associated with poor outcome after AIS, the
effect of acute blood pressure lowering is not clear. Some studies suggest improved,
some unchanged , and others worsened long-term outcomes.](https://image.slidesharecdn.com/intracerebralhemorrhagesahischemicstroke412-230303063307-468e029c/75/Intracerebral-hemorrhage-SAH-ischemic-stroke-412-pptx-46-2048.jpg)






























