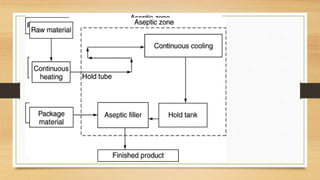
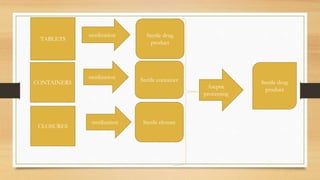
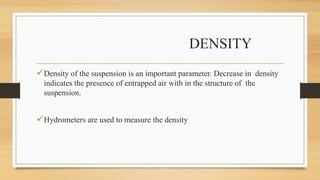
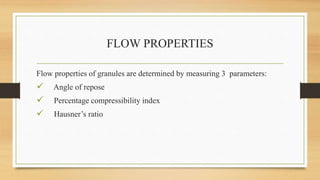
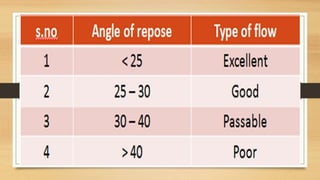
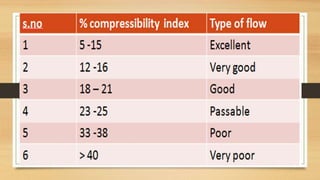
The document provides information on various quality control tests performed during the aseptic processing and manufacturing of different dosage forms including ointments, suspensions, emulsions, powders, and parenterals. Some key tests mentioned are particle size determination, viscosity testing, weight variation, clarity testing, sterility testing, and assays to check for active ingredients and check for uniform drug content. The tests help monitor the quality of products during manufacturing to ensure sterile and stable products are produced.