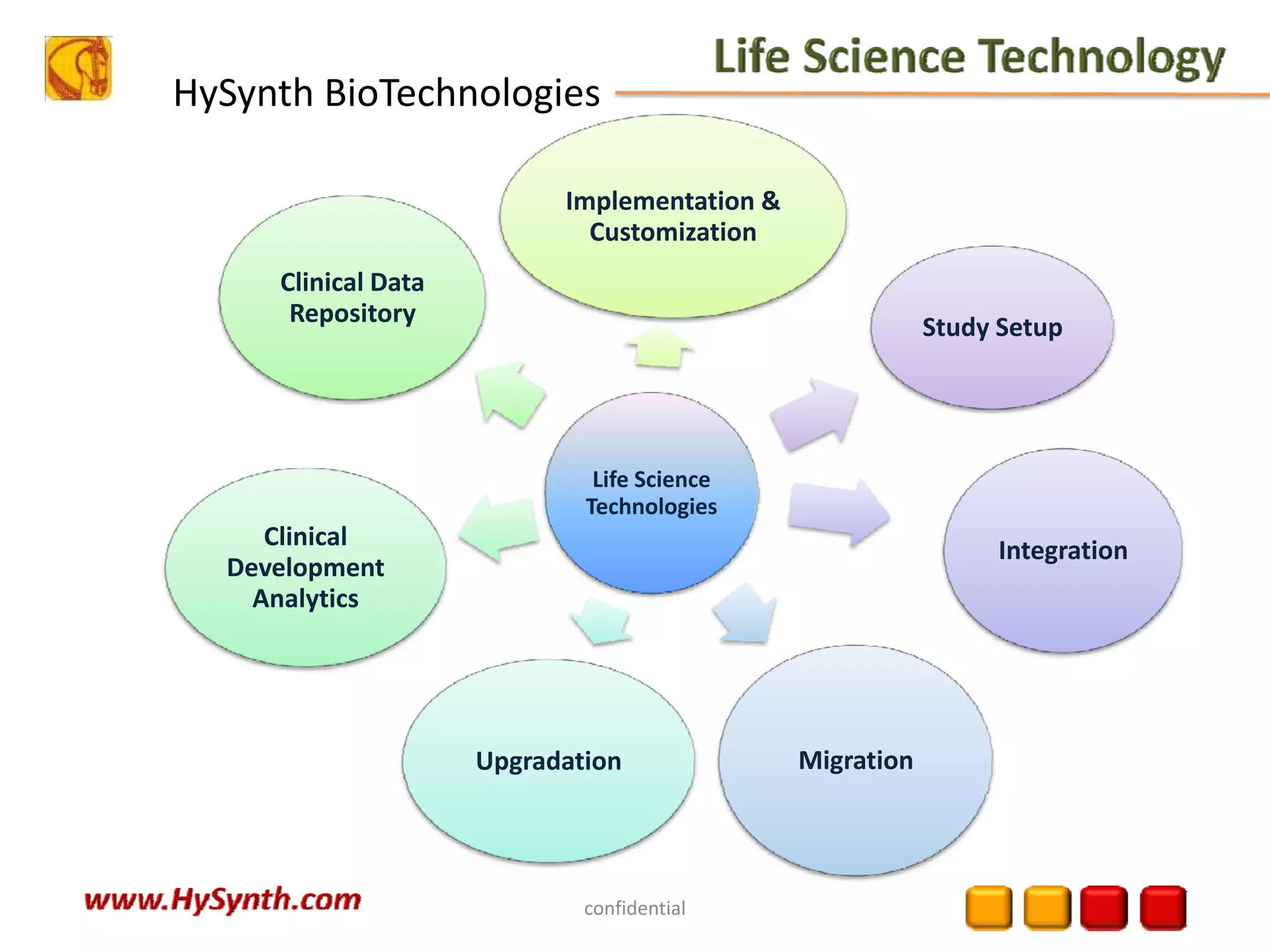
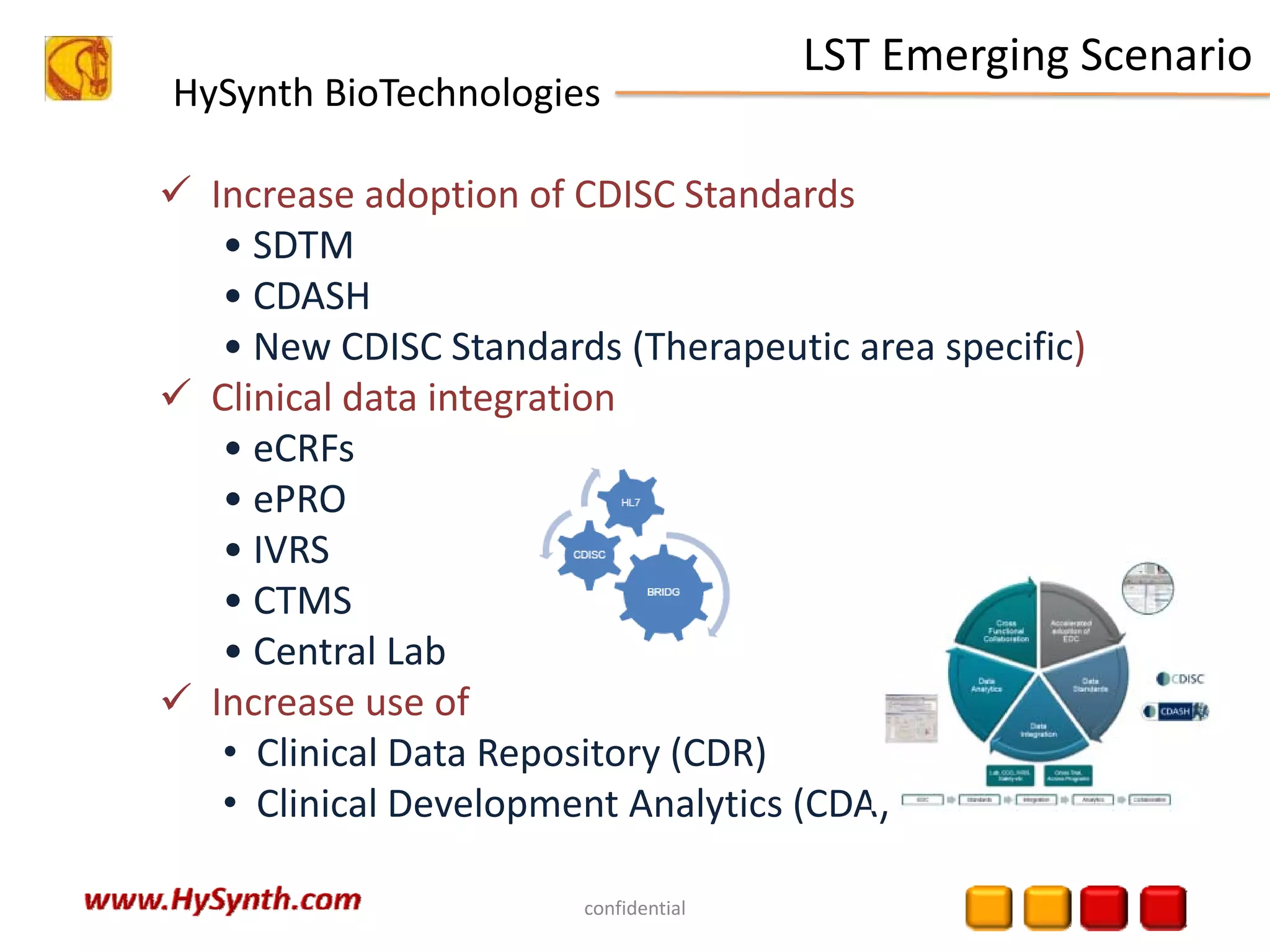
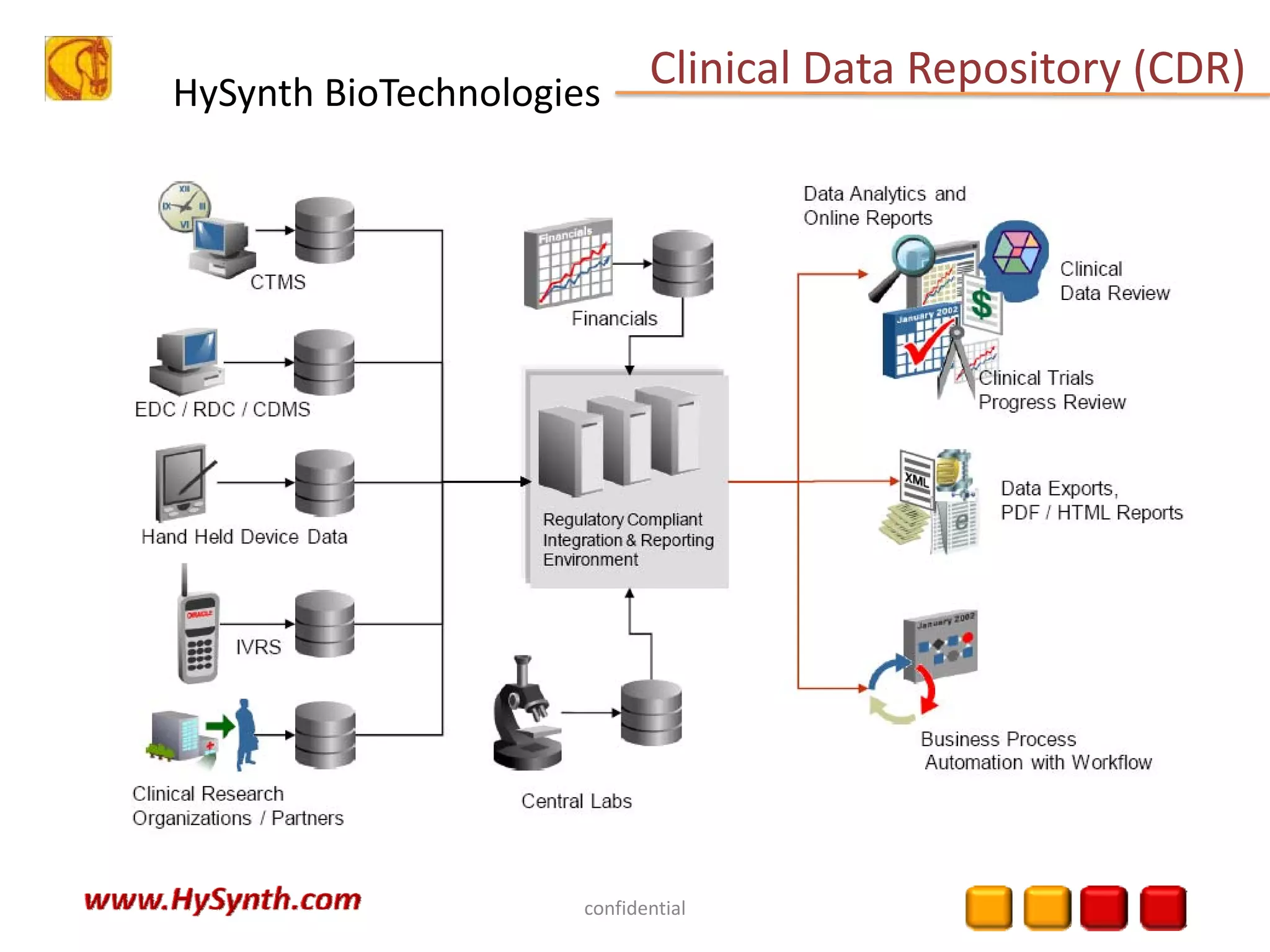
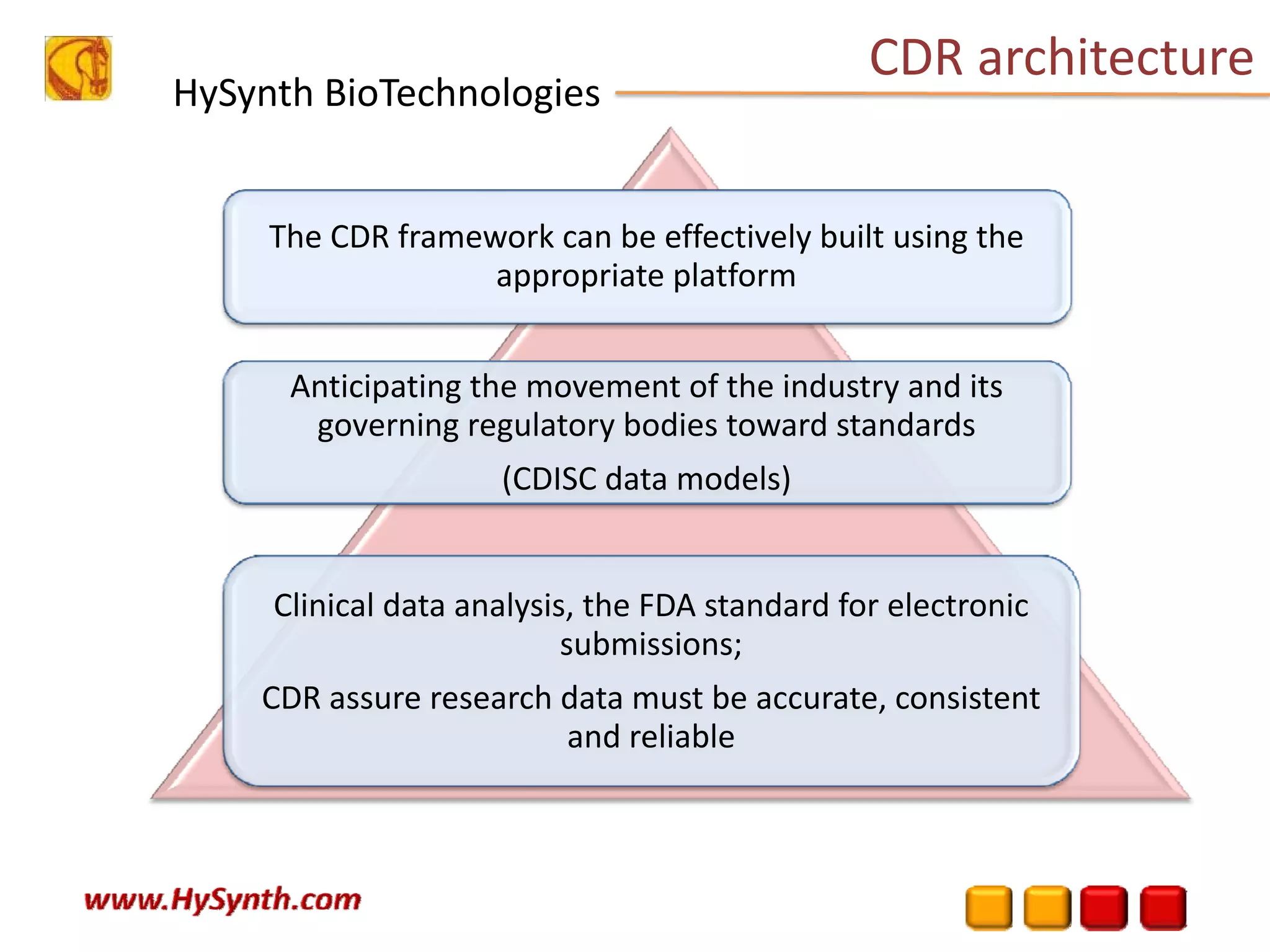
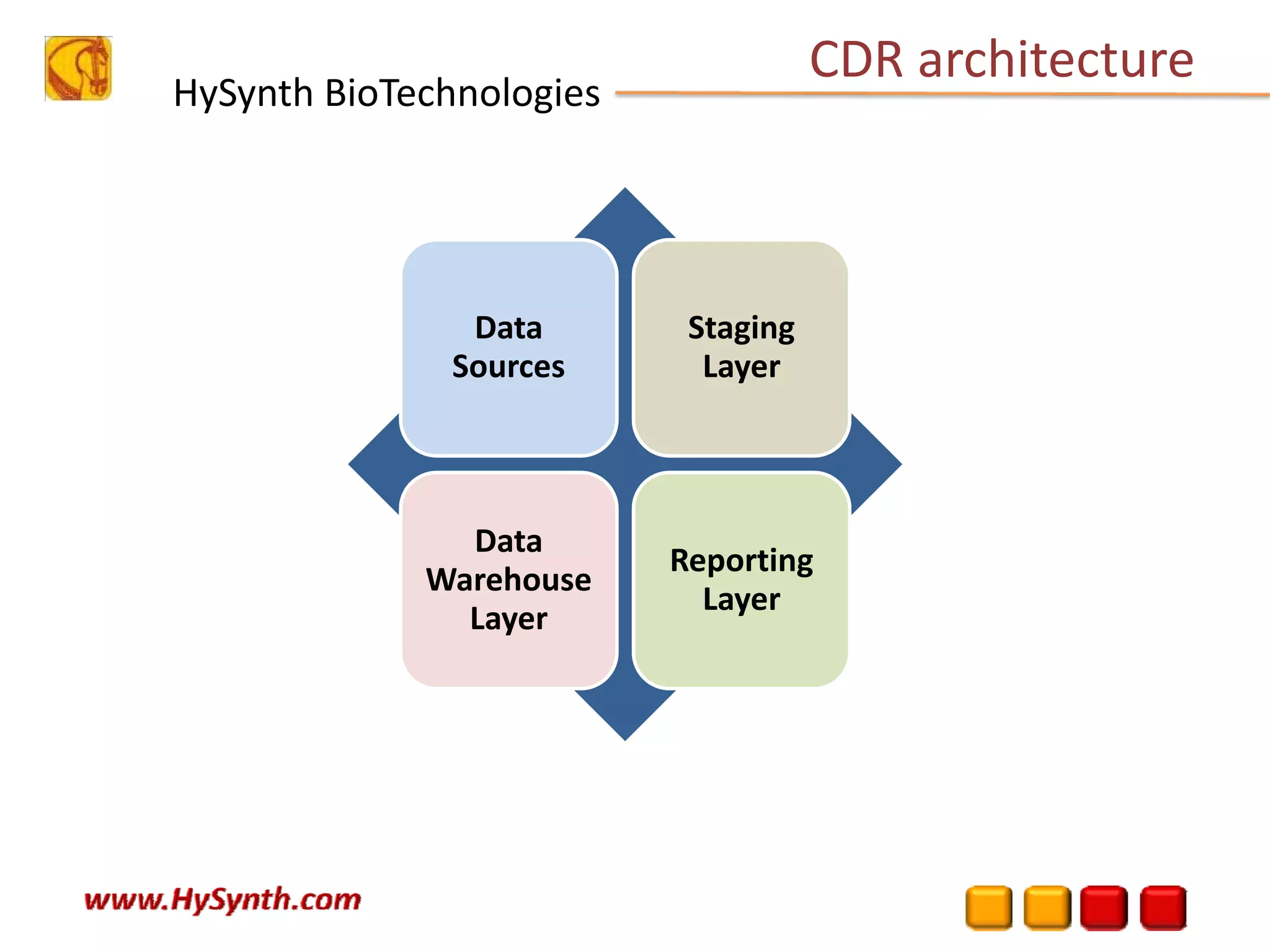
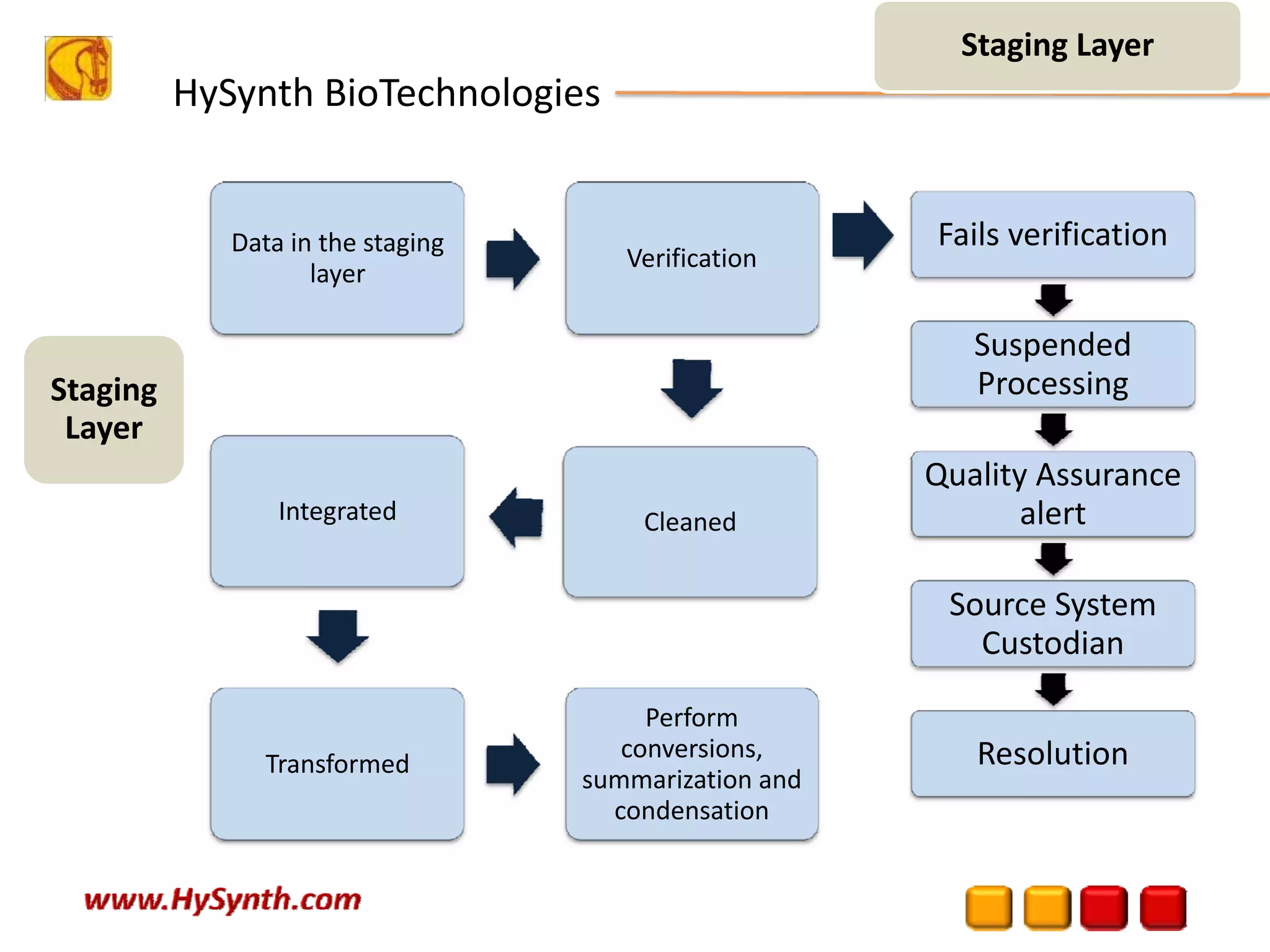
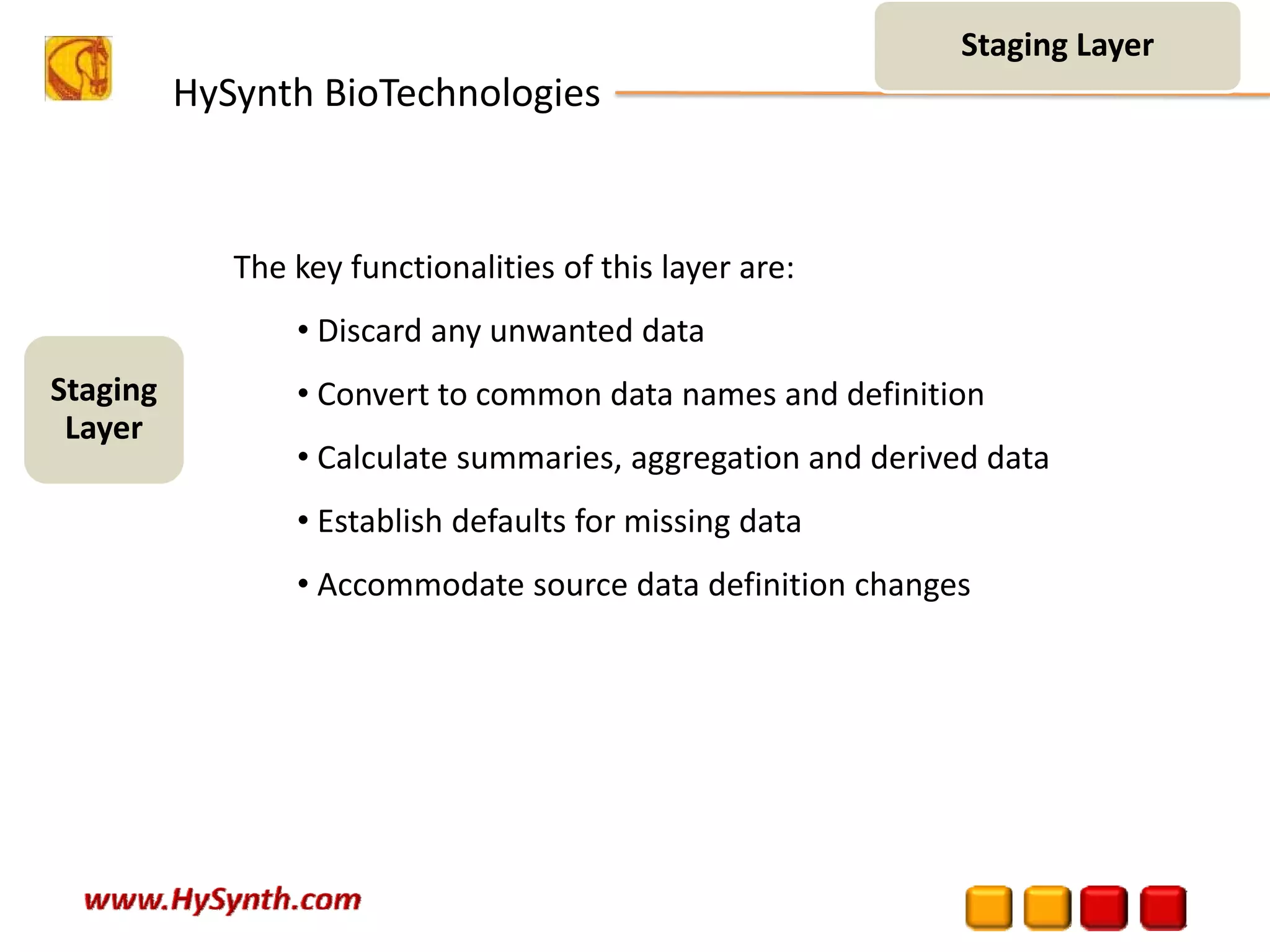
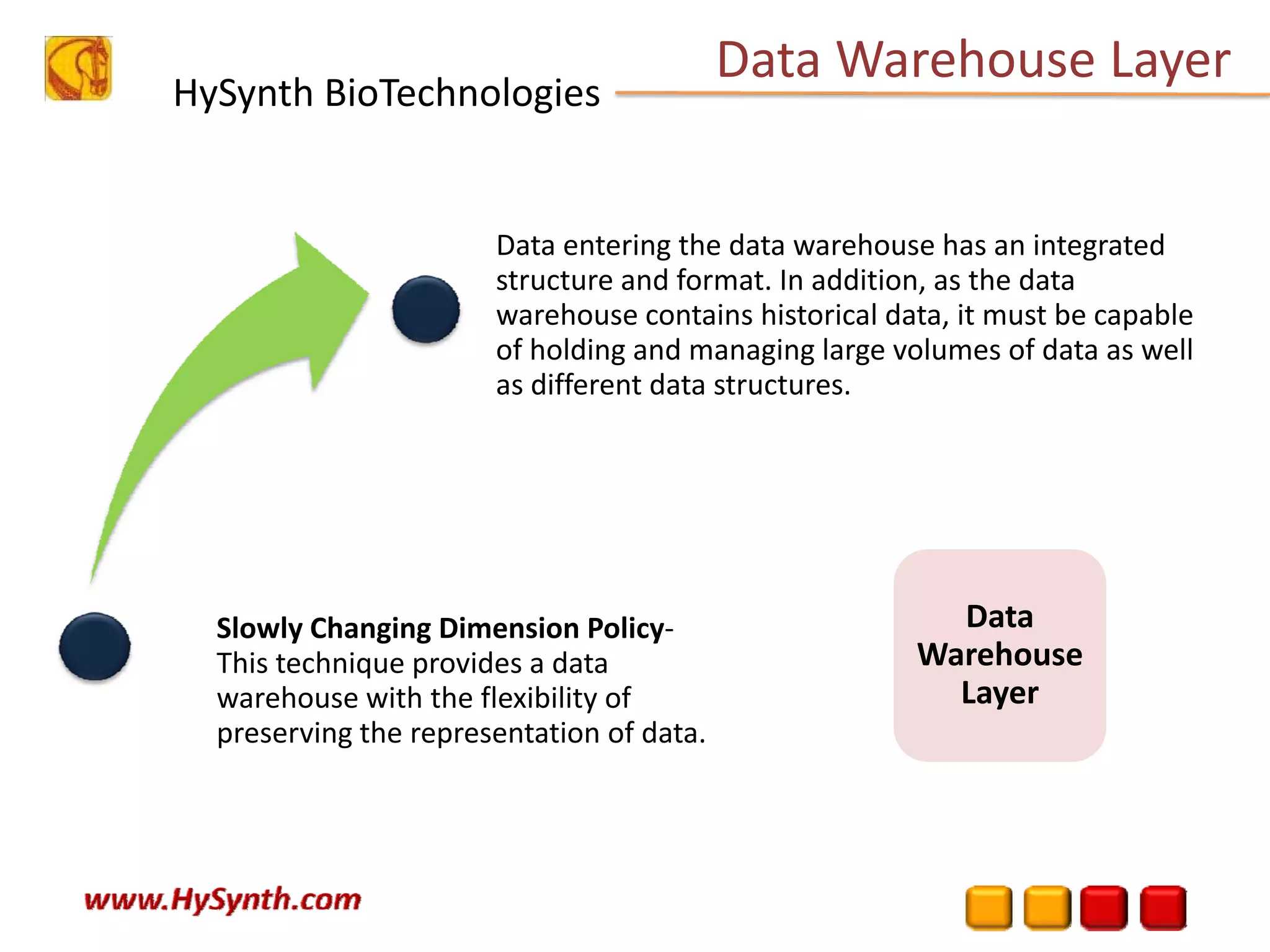
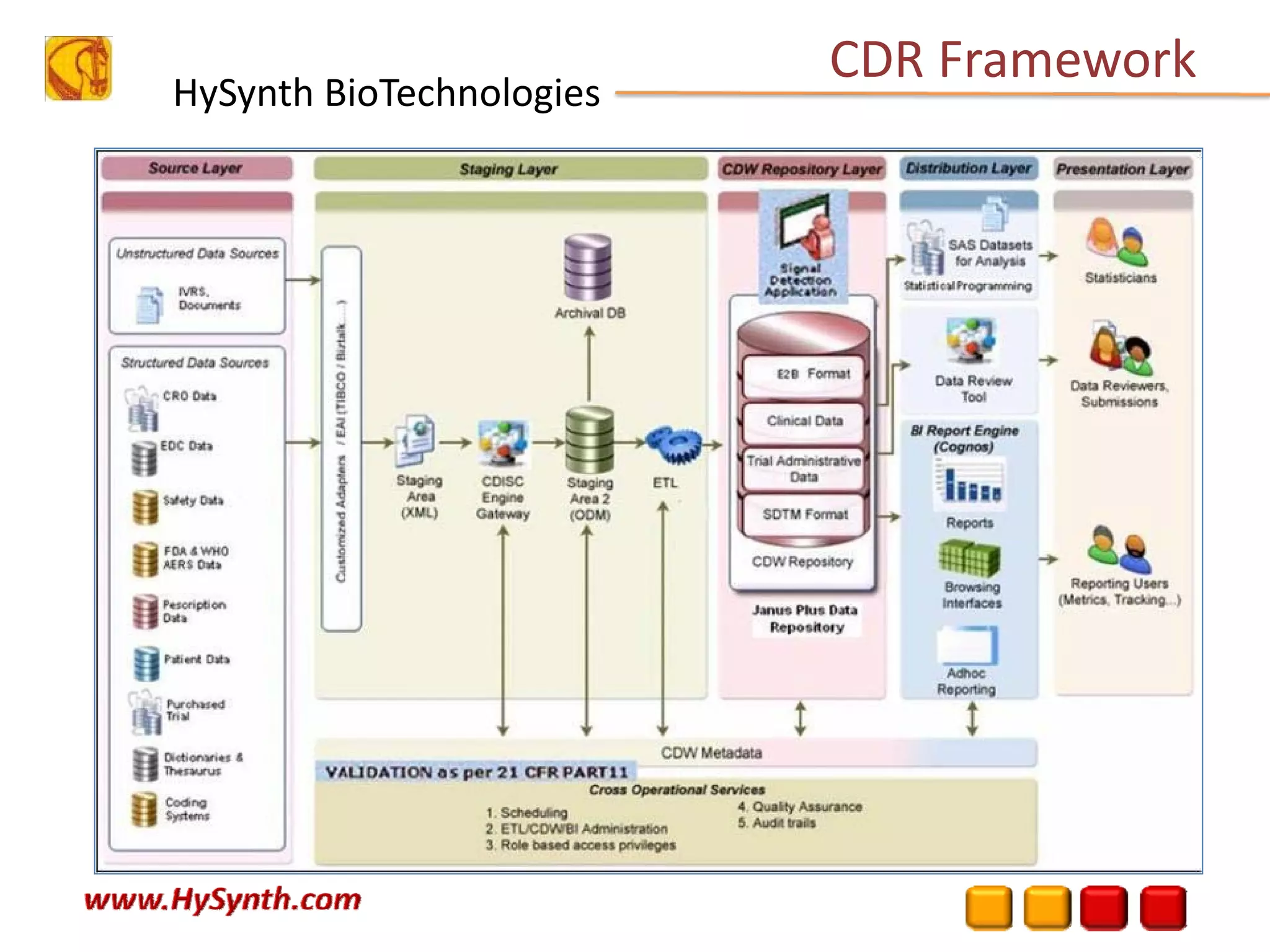
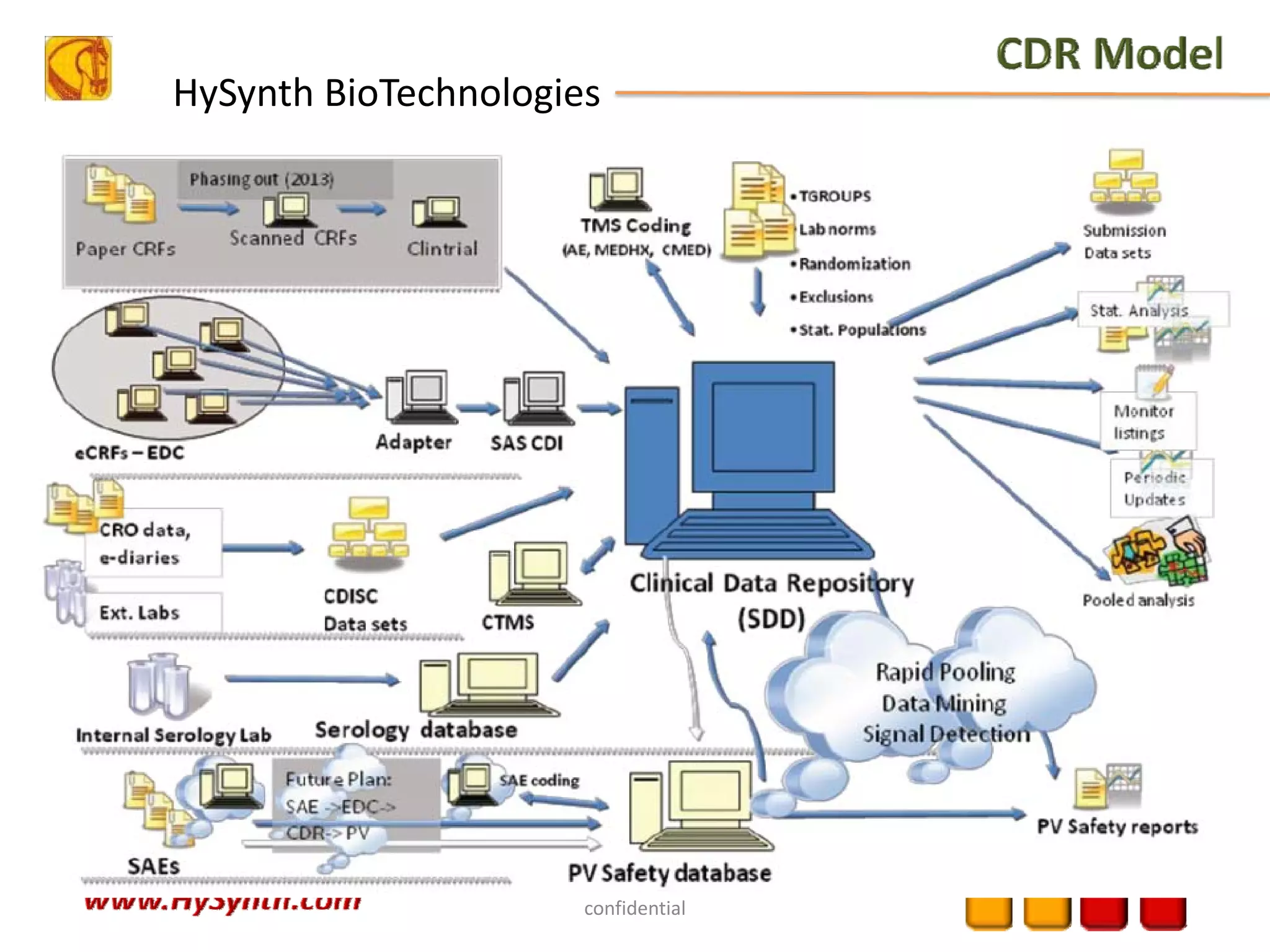
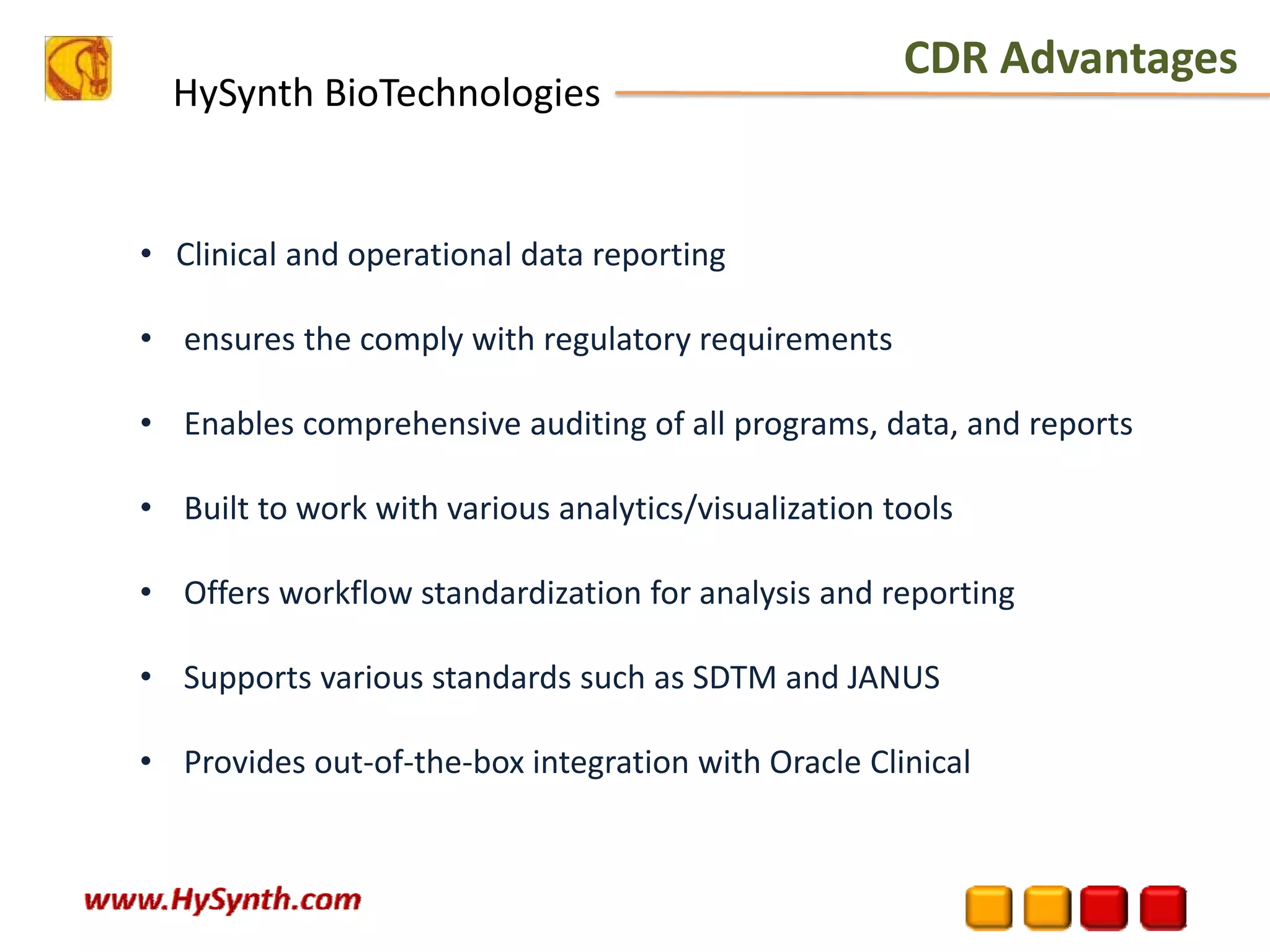
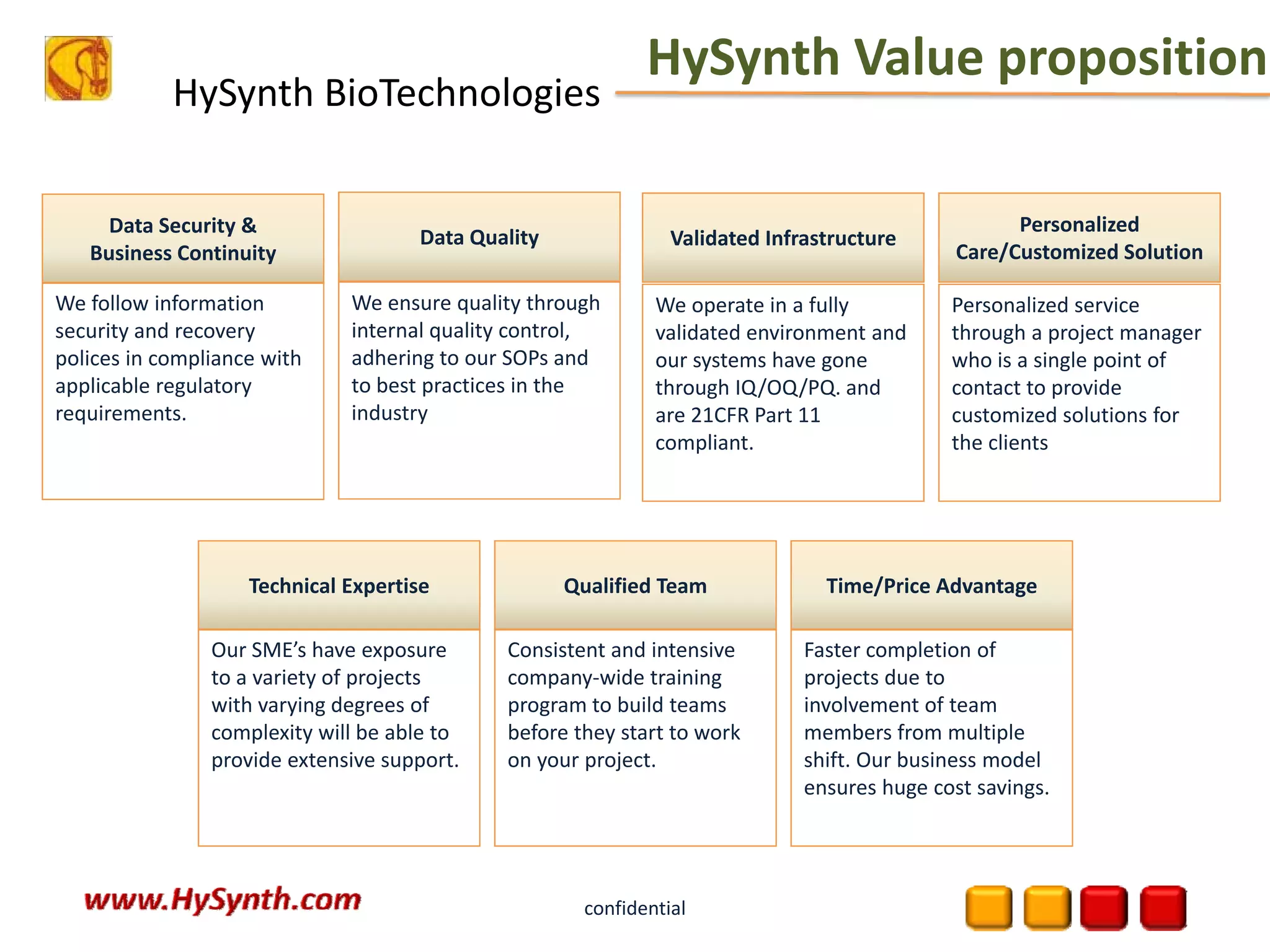
Hysynth Biotechnologies focuses on life science technologies, providing solutions for clinical data management, pharmacovigilance, and biometrics. The company emphasizes the importance of integrating and analyzing data from various sources to improve regulatory compliance and decision-making in clinical trials. They offer a range of services including implementation of clinical data repositories and analytics, ensuring quality assurance and adherence to standards like CDISC.