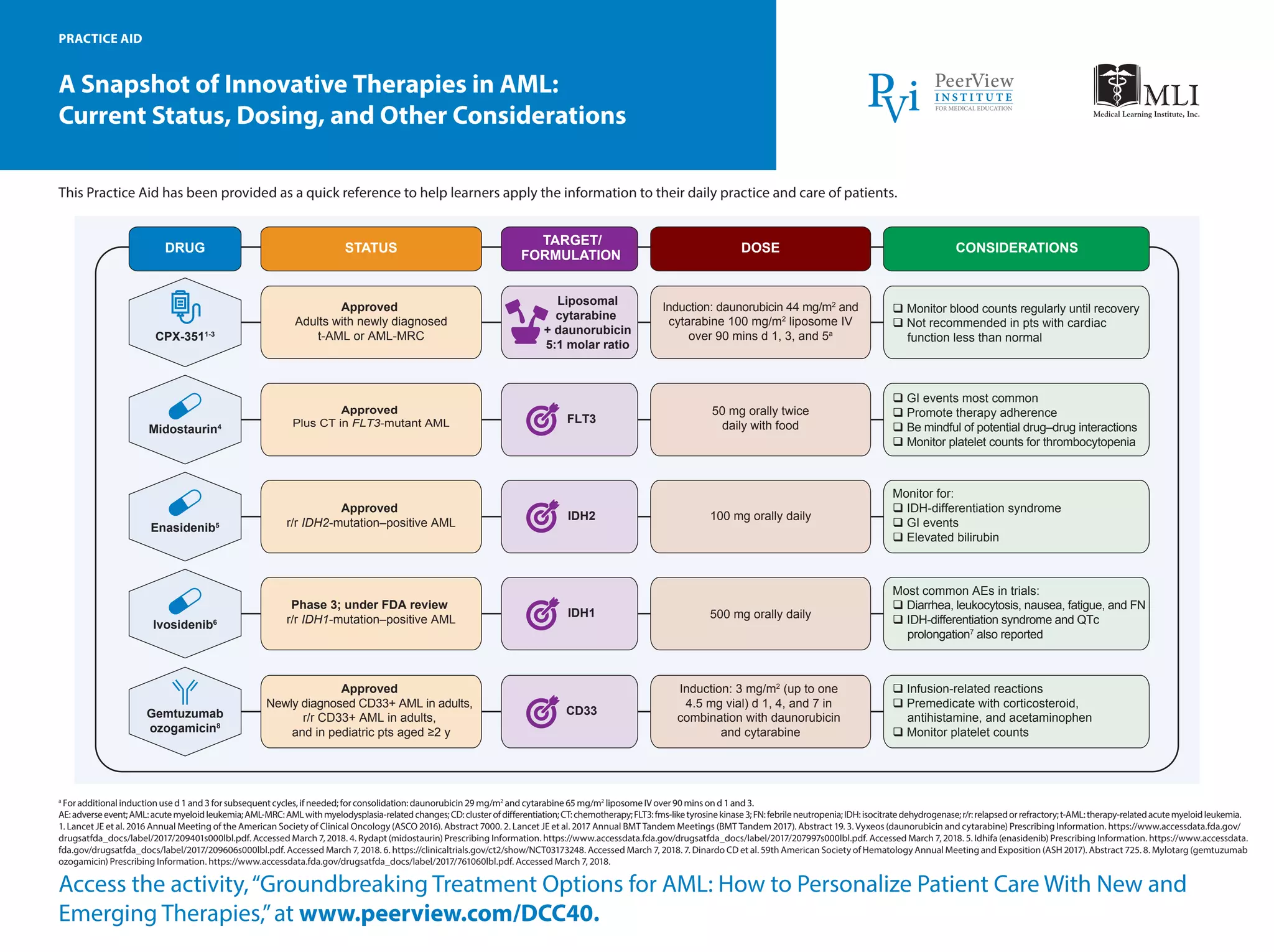
The document provides an overview of innovative therapies for acute myeloid leukemia (AML), detailing the current status, dosing, and considerations for various drugs such as midostaurin, enasidenib, and gemtuzumab ozogamicin. It serves as a practice aid for healthcare providers to assist in patient care and mentions guidelines for monitoring adverse events and drug interactions. Additionally, it includes management strategies and treatment options based on genetic factors and patient age.