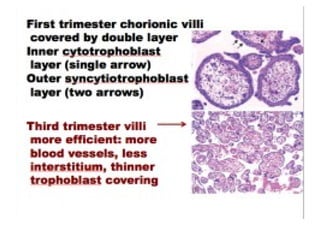
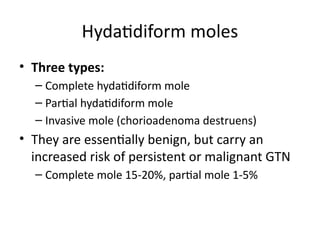
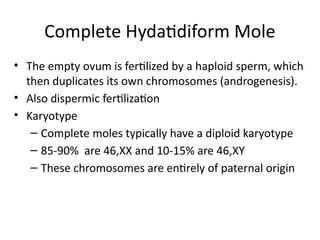
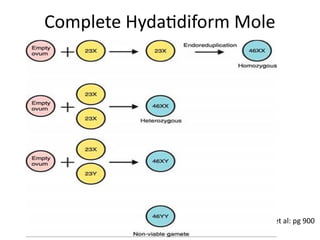
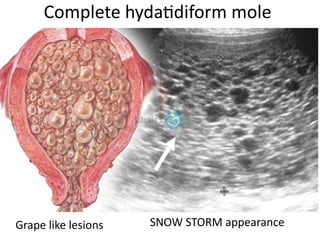
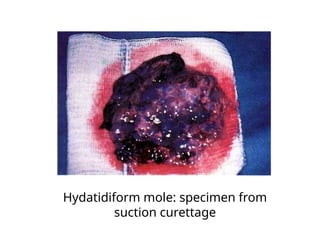
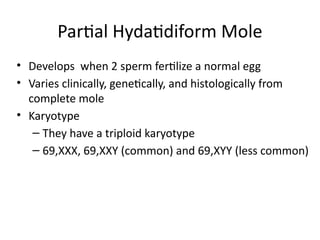
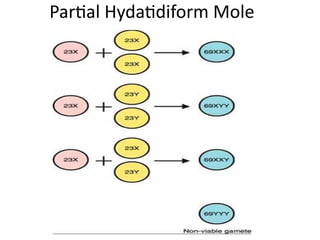
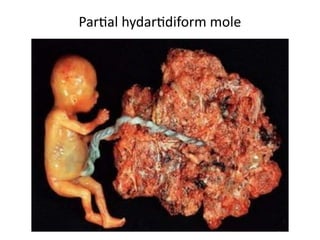
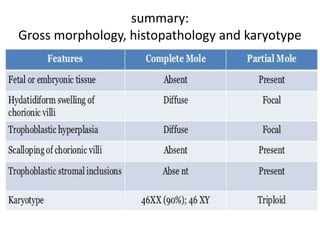
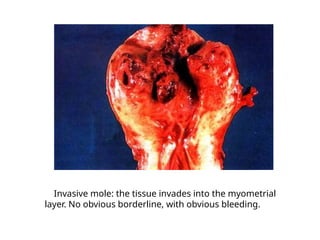
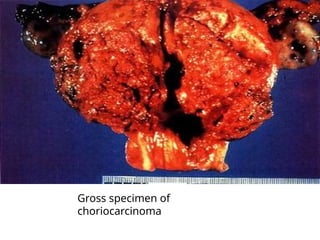
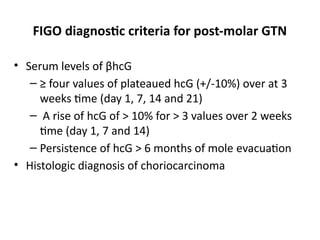
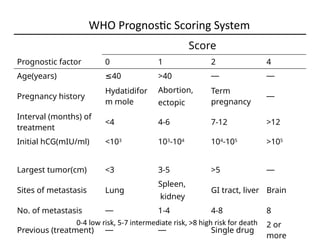
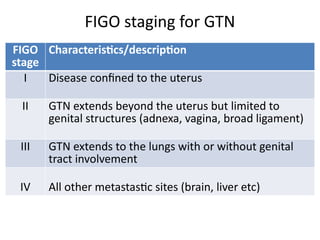
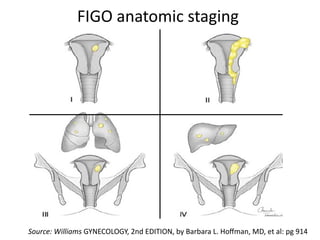
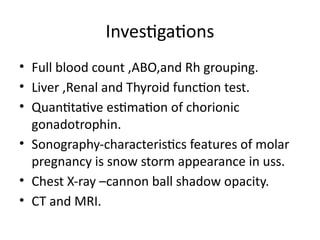
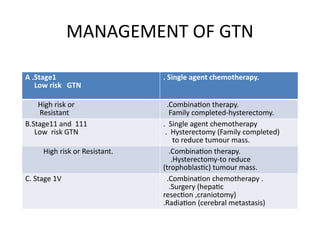
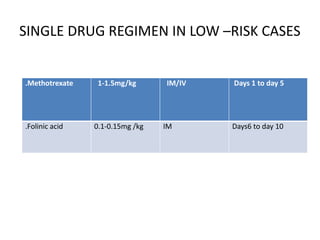
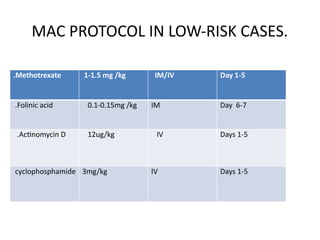
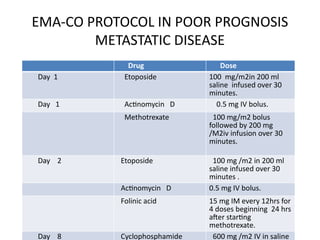
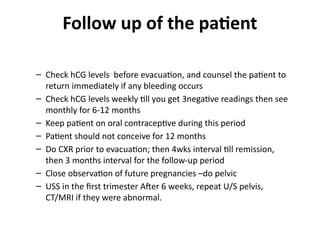
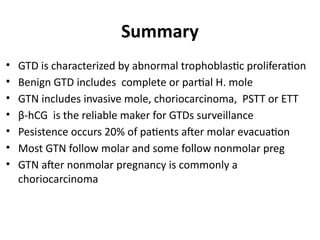
The document provides an overview of gestational trophoblastic diseases (GTDs), which are tumors originating from the placenta and include conditions like hydatidiform moles and gestational trophoblastic neoplasia. It details the epidemiology, risk factors, pathophysiology, treatment options, and diagnostics associated with these diseases. The document emphasizes the importance of β-hCG as a tumor marker and highlights that GTDs can have benign or malignant outcomes, with various treatment regimens depending on the type and stage of the disease.