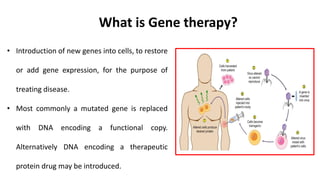
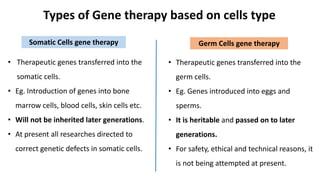
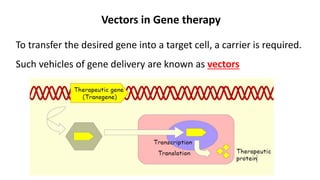
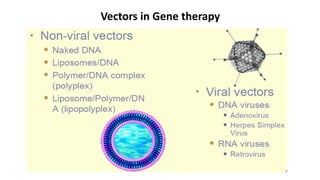
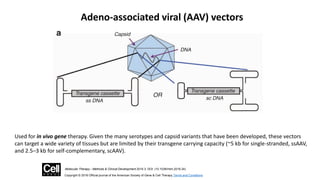
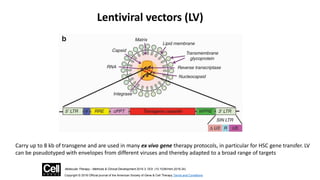
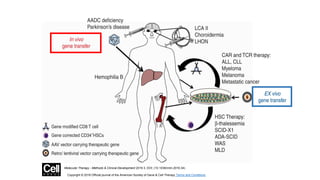
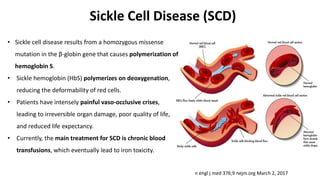
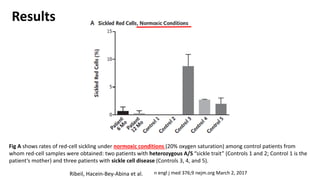
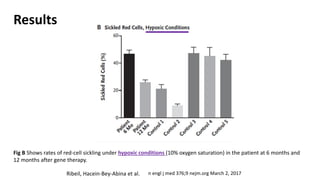
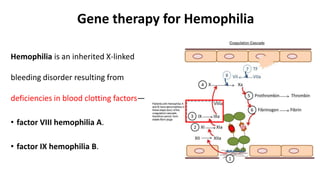
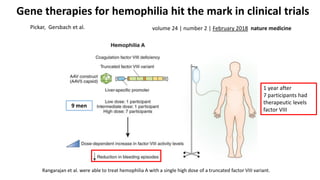
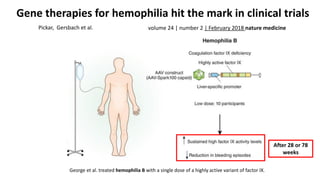
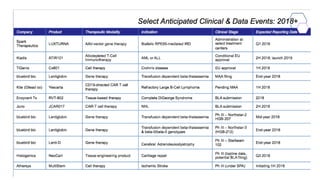
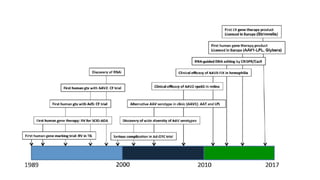
Gene therapy involves introducing new genes into cells to treat disease. Researchers are testing approaches like replacing mutated genes, inactivating genes, or introducing new therapeutic genes. Genes can be transferred into somatic or germ cells using vectors like adeno-associated viral or lentiviral vectors. Examples discussed include successful gene therapy trials for sickle cell disease and hemophilia that showed therapeutic levels of proteins and correction of disease symptoms for over a year. However, challenges remain regarding safe and targeted delivery of genes, immune responses, and ethical issues surrounding gene editing and enhancement.