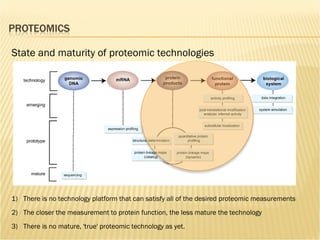
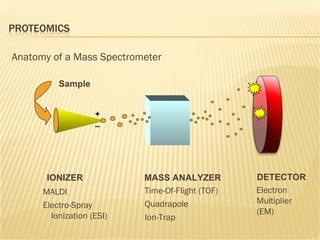
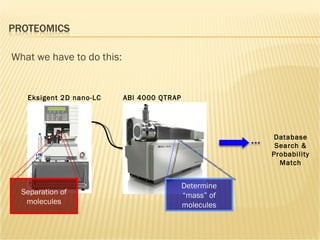
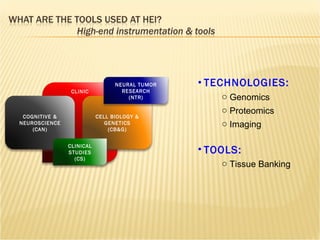
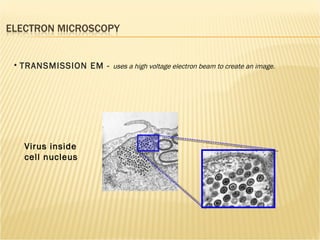
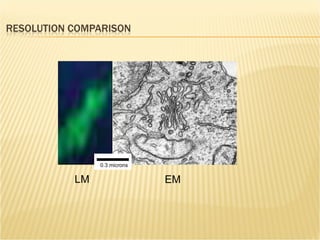
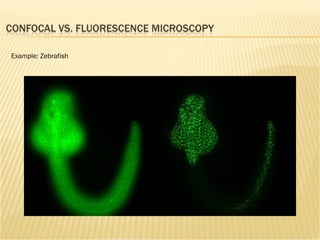
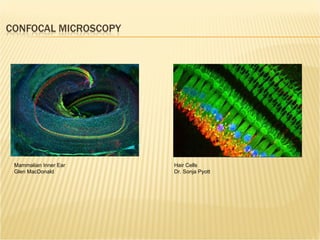
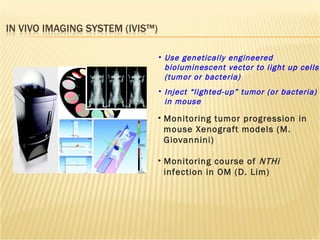
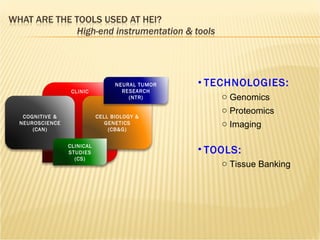
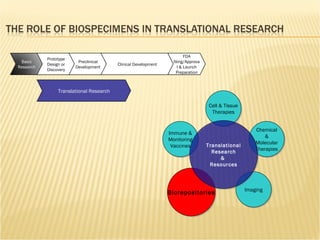
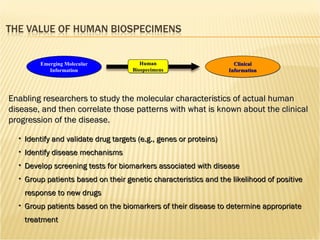
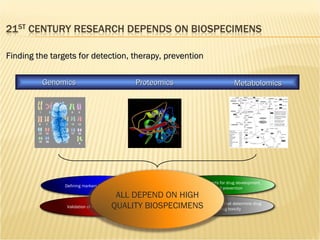
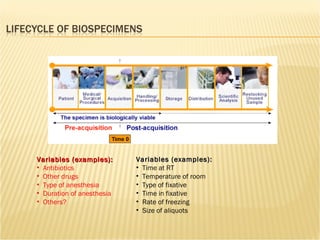
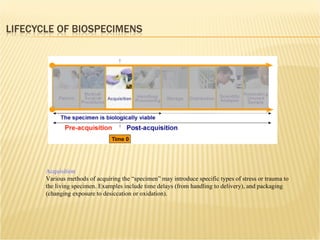
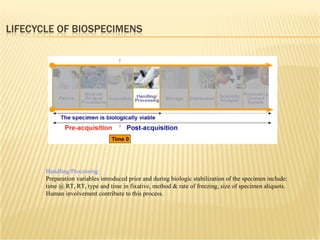
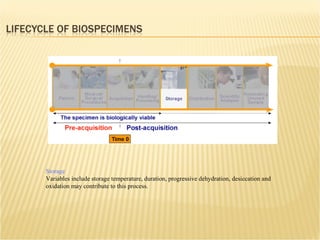
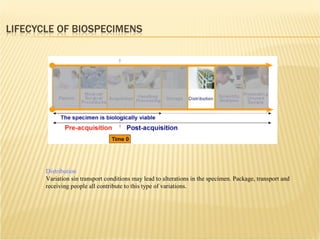
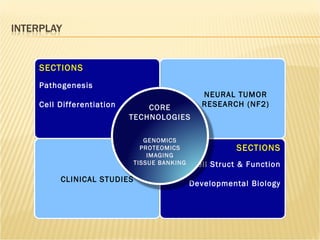
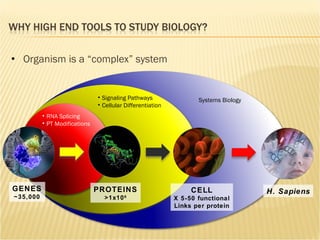
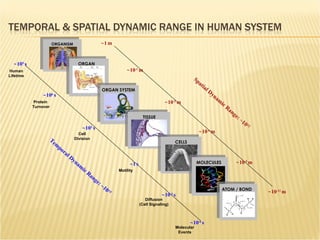
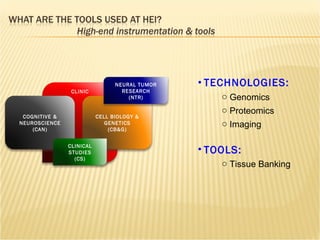
The document discusses various technologies used at the House Ear Institute including genomics, proteomics, and imaging. It describes how researchers are using these tools to study diseases like neurofibromatosis type 2 (NF2) at the molecular level in order to develop personalized treatments and therapies. Maintaining high quality biospecimens is important for enabling various types of research.









![Production of useful protein products for use in medicine, agriculture, bioremediation and pharmaceutical industries. Antibiotics Protein replacement (factor VIII, TPA, streptokinase, insulin, interferon…) BT insecticide toxin (from Bacillus thuringiensis ) Herbicide resistance (glyphosate resistance) Bioengineered foods [e.g. Flavr Savr tomato - delay rotting] “ Pharm” animals](https://image.slidesharecdn.com/gellibolian2010audiovisual2-1270761883288-phpapp01/85/Gellibolian-2010-Audio-Visual2-13-320.jpg)