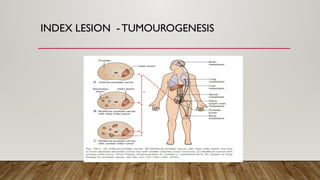
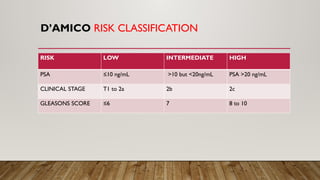
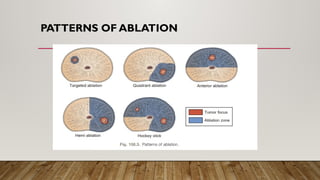
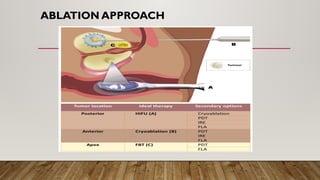
Focal therapy for prostate cancer aims to target only significant cancer foci while preserving surrounding tissue to maintain the patient's quality of life, sexual, and urinary function. With advancements in imaging and biopsy techniques, focal therapy has emerged as an effective alternative to traditional radical treatments, especially for low to intermediate-risk prostate cancer cases. The future of focal therapy hinges on better risk stratification, patient selection, and evaluation of treatment efficacy, with studies showing promising oncologic and functional outcomes.