Embed presentation
Downloaded 15 times







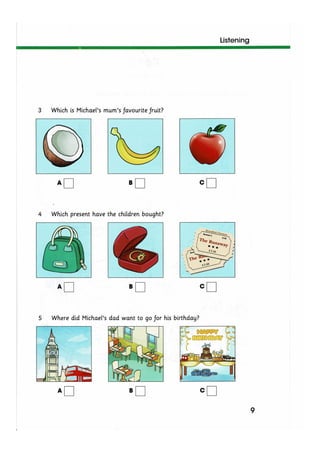




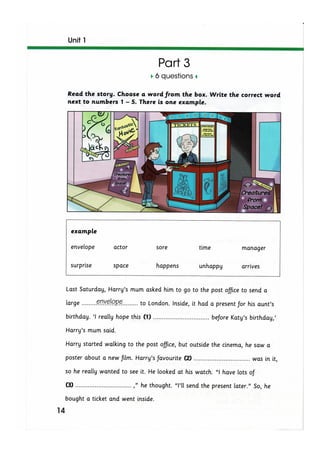

















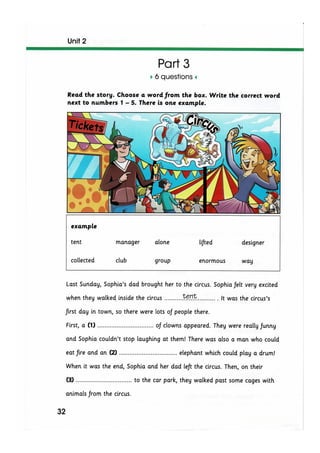





























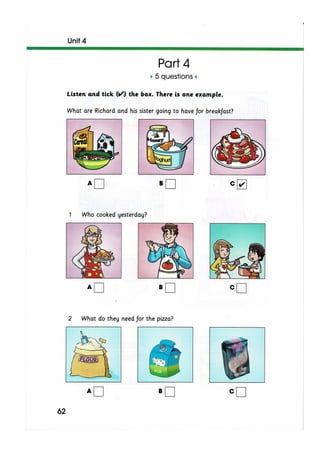























































The document discusses the potential therapeutic medical device (PTMD) review process, which evaluates whether a new medical device provides clinically meaningful advantages over existing legally marketed alternatives for the intended use. The review considers the device's intended use, technological characteristics, unmet medical needs addressed, and available scientific evidence. The PTMD program allows expedited review for certain medical devices that have the potential to provide more effective treatment or enhanced patient care.




















































































































