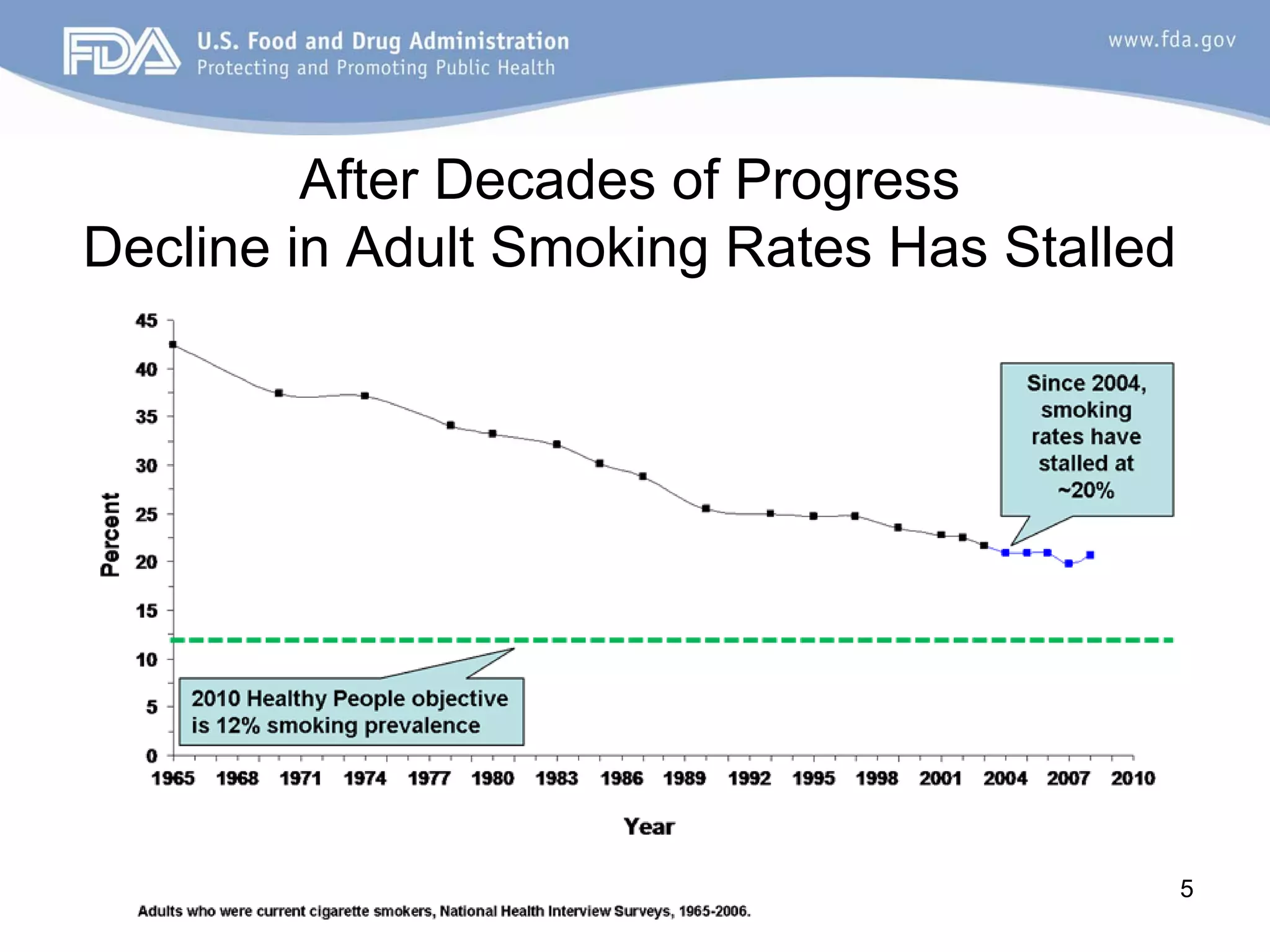
The Center for Tobacco Products (CTP) was established in June 2010 to implement the Family Smoking Prevention and Tobacco Control Act. The CTP aims to reduce tobacco use through preventing youth initiation, promoting cessation, informing consumers, and developing science-based regulations. Tobacco use remains the leading preventable cause of death in the US, killing over 400,000 Americans annually. While adult smoking rates had declined for decades, the rate has now stalled at around 20%. Youth smoking rates have also plateaued despite health initiatives. The CTP will enforce new graphic health warnings, restrictions on misleading terms like "light" and "mild", and potential regulation of modified risk claims. It will continue implementing the Tobacco Control Act to protect public