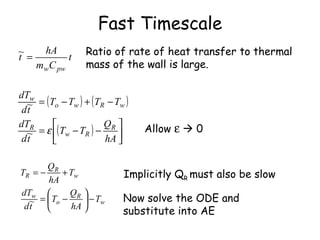
The document discusses using timescale separation to simplify dynamic models with fast and slow processes. It provides two examples: (1) a calcium ion channel model that has fast activation and slower inactivation rates, allowing separation into fast and slow timescales; (2) a reactor wall heat transfer model where the wall thermal mass is small compared to the reactor, allowing separation based on the fast temperature response of the wall and slower temperature change in the reactor. Both examples non-dimensionalize variables, identify a small parameter epsilon as the ratio of timescales, then take the fast timescale limit of epsilon to zero to obtain simplified equations representing each timescale.



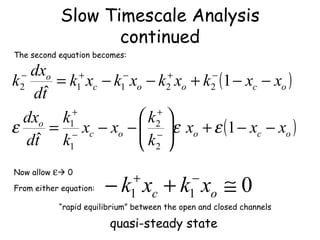




![Heat Transfer Continued Rearrange the Equations for T w and T R [time] -1 Slow time scale Transfer Rate across wall small compared to the thermal mass of reactor](https://image.slidesharecdn.com/fastslowtimescaleanalysis-1-090311152217-phpapp02/85/Fast-Slow-Timescale-Analysis-1-10-320.jpg)