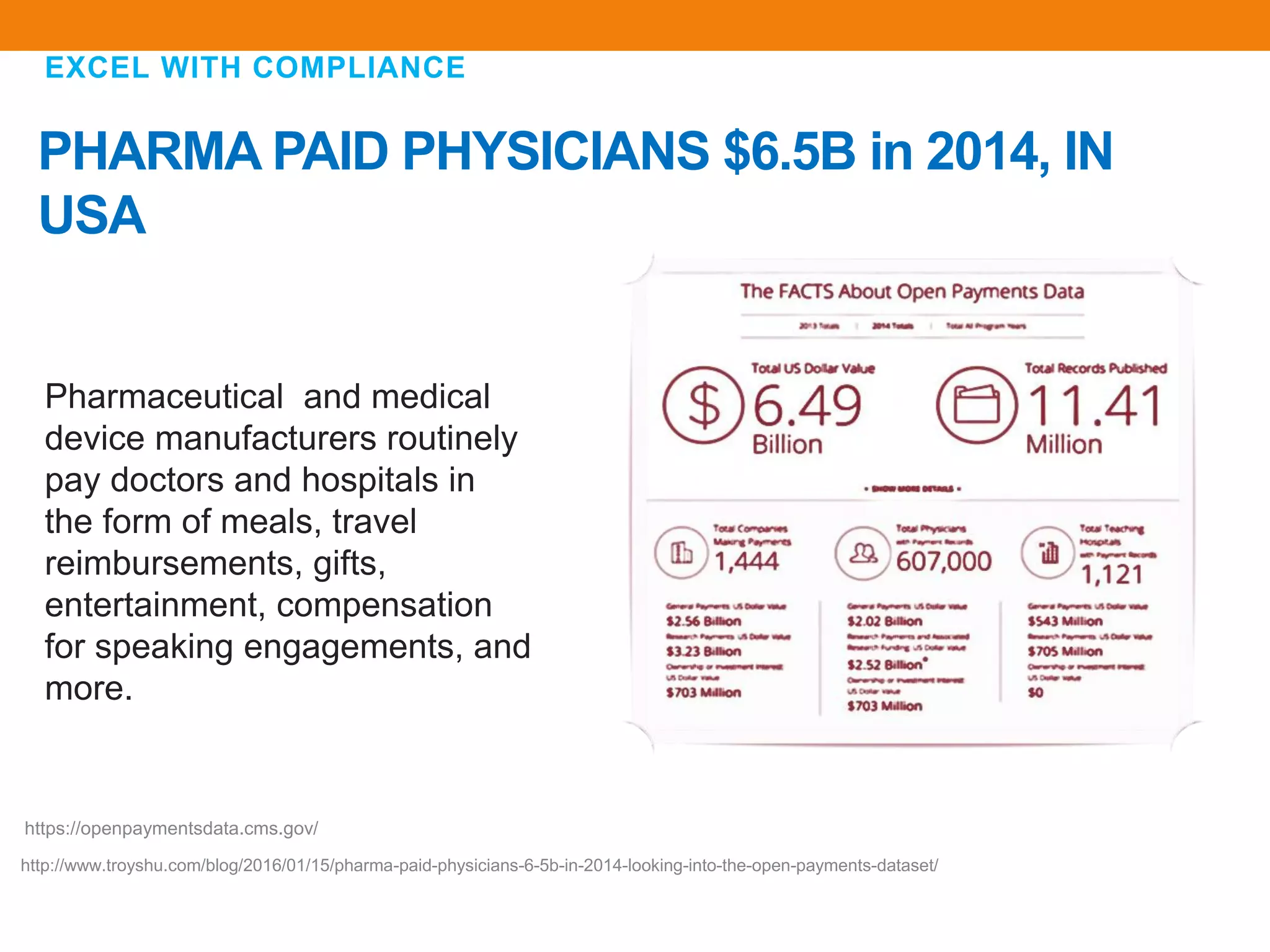
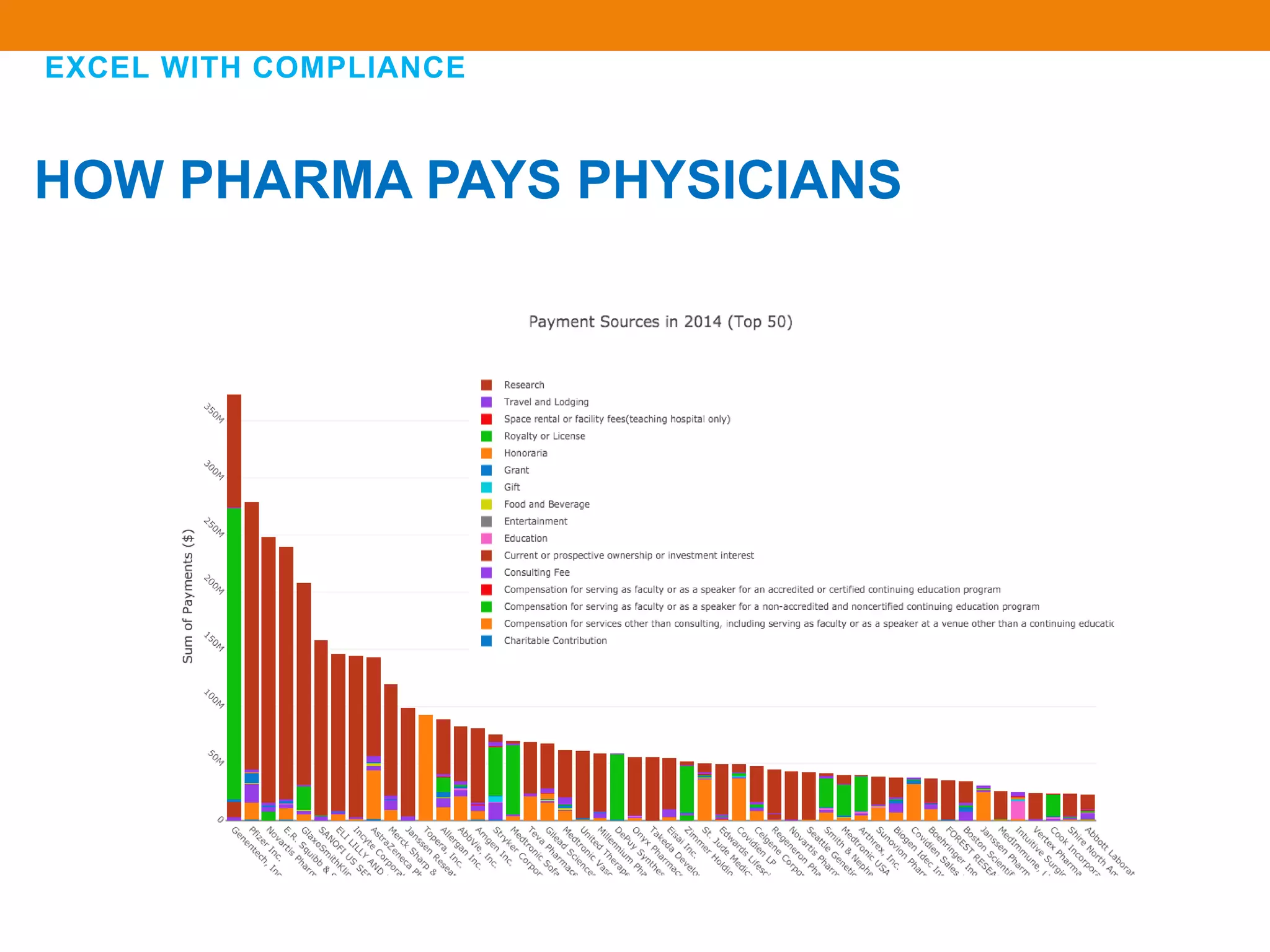
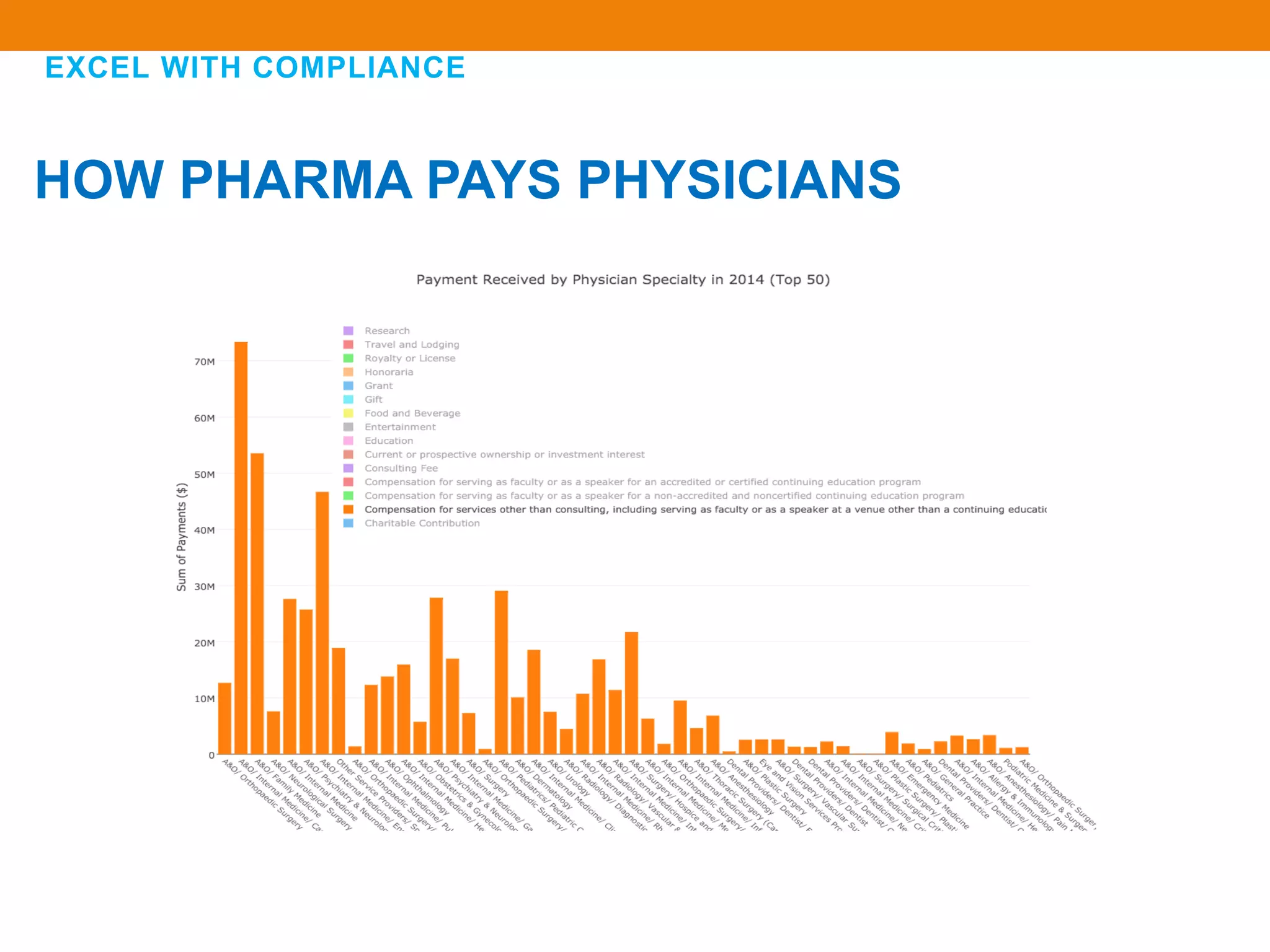
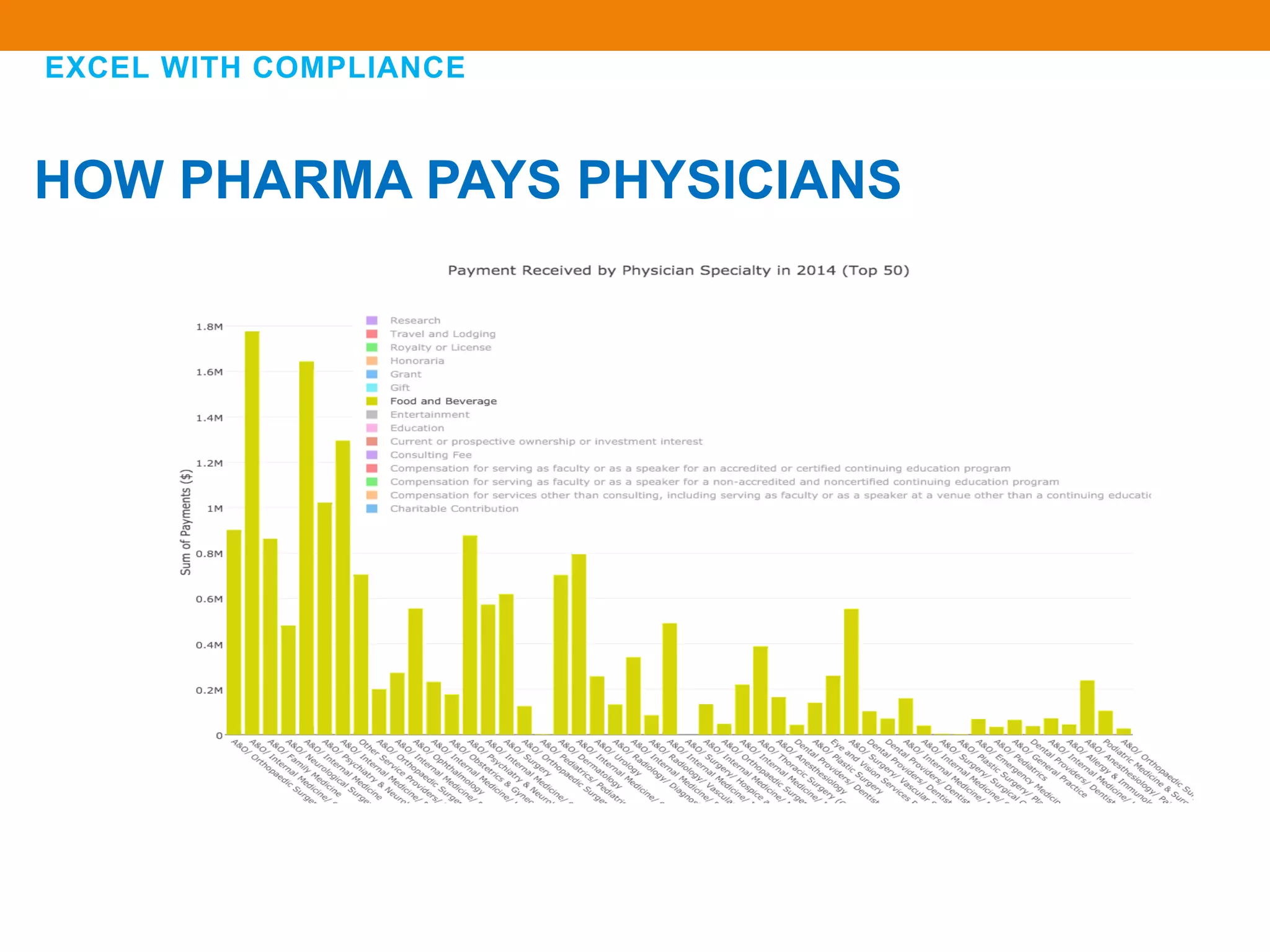
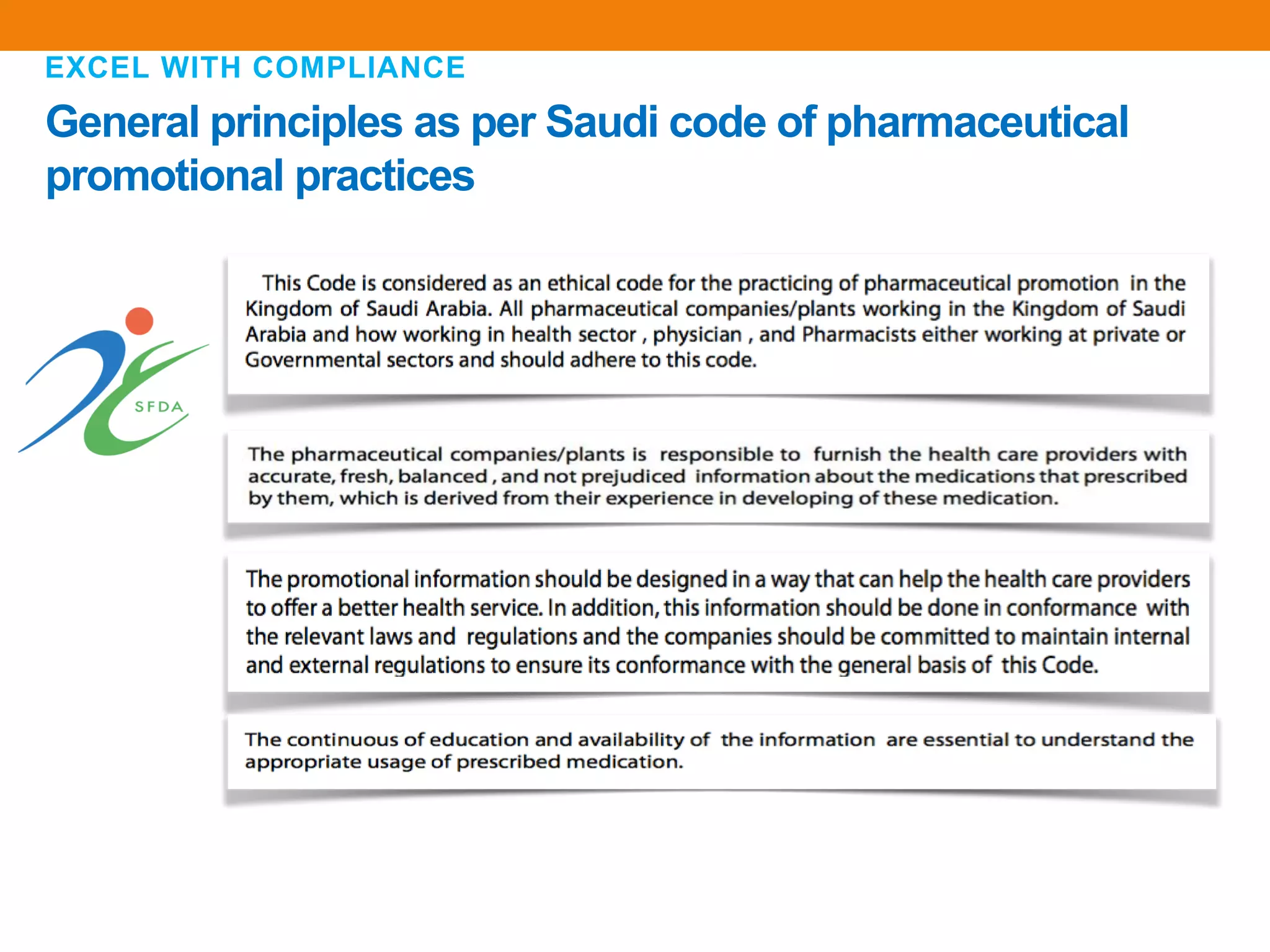
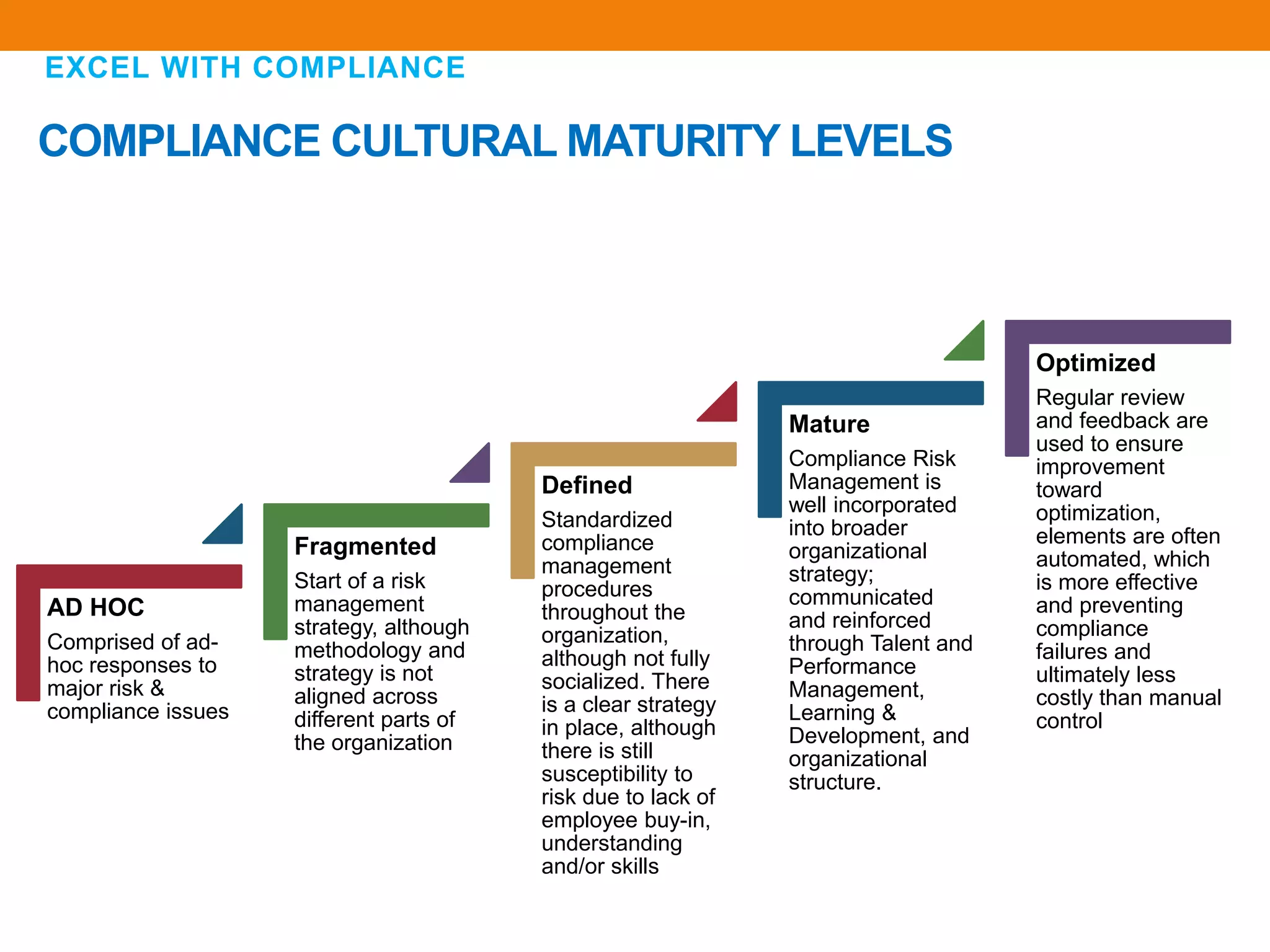
The document discusses compliance in the pharmaceutical industry, particularly in the context of Saudi Arabia, detailing regulations governing promotional practices, ethical considerations, and responsibilities for healthcare providers (HCPs). It emphasizes the importance of creating a compliant culture through structured risk assessment, training, and accountability, while outlining dos and don'ts for pharmaceutical promotions and interactions with HCPs. Additionally, it addresses the financial aspects of how the pharmaceutical industry compensates physicians and stresses the need for adherence to local laws and ethical standards.