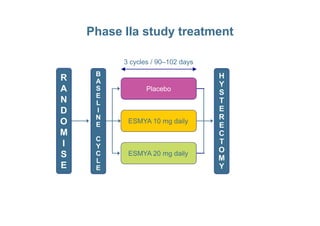
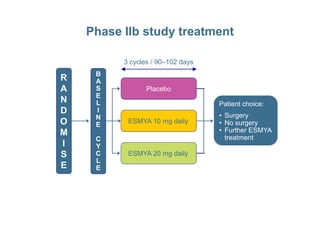
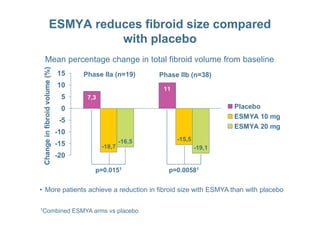
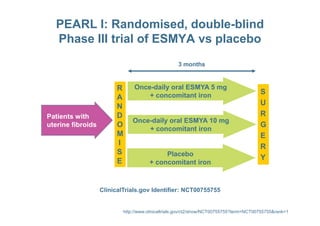
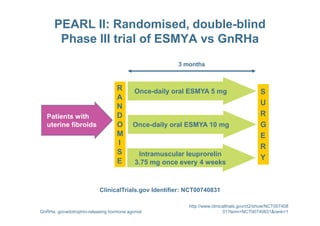
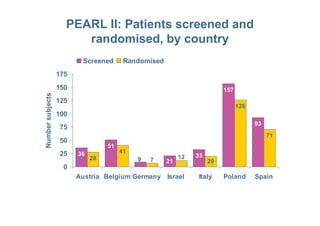
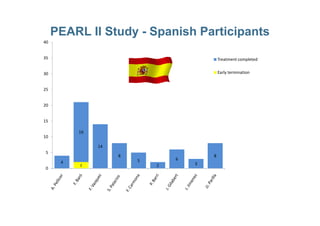
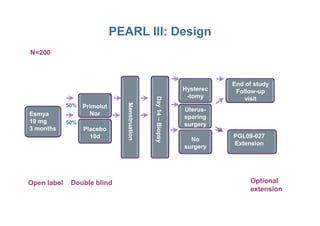
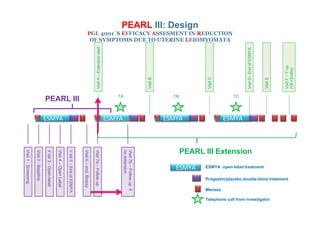
This document provides information on ESMYA (ulipristal acetate), a selective progesterone receptor modulator being studied for the treatment of uterine fibroids. It summarizes results from Phase II and Phase III clinical trials that showed ESMYA significantly reduced fibroid size and bleeding compared to placebo. The Phase III PEARL I and PEARL II trials are further evaluating ESMYA's efficacy and safety profile compared to placebo or a gonadotropin-releasing hormone agonist. Additional trials are exploring long-term use of ESMYA and its effects on bleeding patterns, endometrial histology, and potential for uterus-sparing treatments.