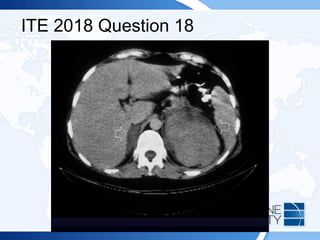
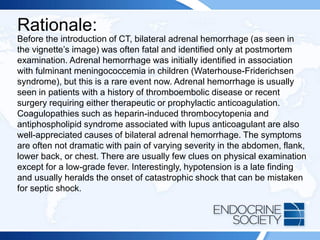
A 54-year-old man with a history of back pain developed Cushing syndrome symptoms after receiving corticosteroid injections. Diagnostic tests indicated iatrogenic Cushing syndrome due to triamcinolone acetonide, which he received for pain management. Additionally, a 74-year-old man post-hip replacement showed signs leading to a diagnosis of bilateral adrenal hemorrhage linked to anticoagulation therapy.


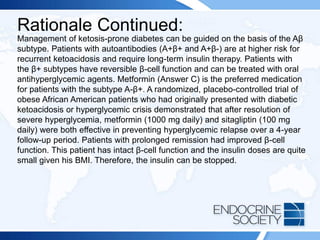
![ITE 2018 Question 7
A 54-year-old man is referred for evaluation of fatigue and possible Cushing
syndrome. His problems started after a back injury at work 1 year ago. He
developed sudden lower back pain while lifting a heavy load and was sent
home. He was evaluated and prescribed physical therapy for 6 weeks with
some relief, and then he was given monthly back injections in a pain clinic
for 6 months (the last treatment was 4 months ago). The injections markedly
eased his pain, but he developed rapid weight gain, hunger, easy bruising,
and facial fullness. His initial evaluation with his primary care physician
documented the following laboratory values:
• Plasma ACTH = 4 pg/mL (10-60 pg/mL) (SI: 0.9 pmol/L [2.2-13.2 pmol/L])
• Urinary free cortisol = <3 µg/24 h (4-50 µg/24 h) (SI: <8.3 nmol/d [11-138
nmol/d])
• Overnight 1-mg dexamethasone suppression test, cortisol = <0.2 µg/dL (SI: <5.5
nmol/L)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-3-320.jpg)















![ITE 2018 Question 37
Laboratory test results:
• Sodium = 138 mEq/L (136-142 mEq/L) (SI: 138 mmol/L [136-142 mmol/L])
• Potassium = 4.8 mEq/L (3.5-5.0 mEq/L) (SI: 4.8 mmol/L [3.5-5.0 mmol/L])
• Serum DHEA-S = <15 µg/dL (44-332 µg/dL) (SI: <0.4 µmol/L [1.19-9.11 µmol/L])
• Serum testosterone = <20 ng/dL (8-60 ng/dL) (SI: <0.7 nmol/L [0.3-2.1 nmol/L])
• Plasma renin activity = 3.4 ng/mL per h (0.6-4.3 ng/mL per h)
• Serum androstenedione = 30 ng/dL (80-240 ng/dL) (SI: 1.0 nmol/L [2.79-8.38
nmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-25-320.jpg)





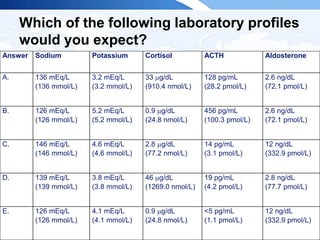
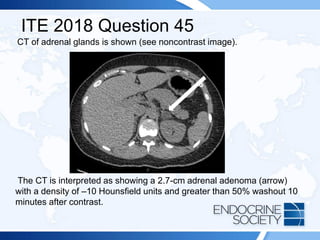
![ITE 2018 Question 45
Laboratory test results:
• Cortisol (8 AM, after 1 mg overnight dexamethasone suppression test) =
1.1 μg/dL (SI: 30.3 nmol/L)
• Metanephrines (plasma fractionated)
o Metanephrine = 22 pg/mL (<57 pg/mL) (SI: 111.5 pmol/L [<289
pmol/L])
o Normetanephrine = 112 pg/mL (<148 pg/mL) (SI: 611.5 pmol/L [<808
pmol/L])
• Aldosterone = 10 ng/dL (1-21 ng/dL) (SI: 277.4 pmol/L [27.7-582.5
pmol/L])
• Plasma renin activity = 1.4 ng/mL per h (0.6-4.3 ng/mL per h)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-31-320.jpg)



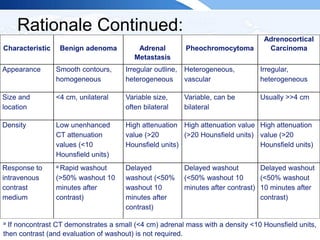




![ITE 2018 Question 47
Initial laboratory test results (while taking lisinopril, hydrochlorothiazide, and
amlodipine):
• Morning serum aldosterone = 19 ng/dL (1-21 ng/dL) (SI: 527.1 pmol/L
[27.7-582.5 pmol/L])
• Plasma renin activity = 0.2 ng/mL per h (0.6-4.3 ng/mL per h)
• Urinary aldosterone = 18 µg/24 h
• Urinary sodium = 202 mEq/24 h (40-217 mEq/24 h) (SI: 202 mmol/d [40-
217 mmol/d])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-41-320.jpg)
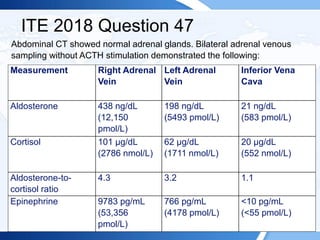



![ITE 2018 Question 53
A 36-year-old woman has a 10-year history of anxiety and depression. She
has been treated with venlafaxine for 5 years with substantial improvements
in her anxiety, but over the last year she has developed more frequent
anxiety episodes and occasional palpitations. Alprazolam was prescribed
several weeks ago, but it has not relieved her symptoms. During a routine
primary care appointment, her blood pressure was 162/90 mm Hg, her pulse
rate was 102 beats/min, and she was noted to be anxious. Her primary care
physician ordered thyroid function tests, which were normal, and then
ordered measurement of plasma metanephrines to evaluate whether she
could have a catecholamine-producing tumor contributing to her anxiety.
• Plasma normetanephrine = 222 pg/mL (<148 pg/mL) (SI: 1212.1 pmol/L
[<808 pmol/L])
• Plasma metanephrine = 80 pg/mL (<57 pg/mL) (SI: 405.6 pmol/L [<289
pmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-47-320.jpg)


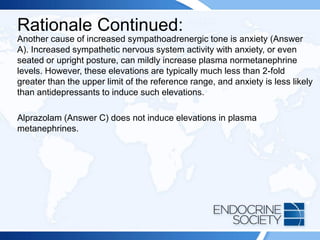
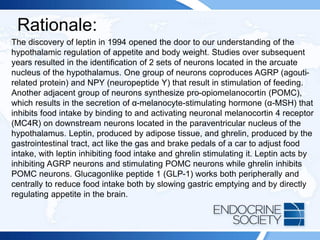
![Rationale Continued:
An increasingly common cause of false-positive test results in this setting is
the use of medications such as tricyclic antidepressants, serotonin
reuptake inhibitors, and norepinephrine and/or epinephrine reuptake
inhibitors. These medications (such as venlafaxine [Answer B]) can induce
mild elevations in plasma normetanephrine and metanephrine that are
usually less than 2-fold greater than the upper limit of the reference range;
however, in rare cases these medications can induce greater than 2-fold
elevations in metanephrines. This suspicion is difficult to confirm since
stopping the medication may not be safe from a psychiatric view point and
it can take 6 to 8 weeks after medication cessation for metanephrines to
normalize. A clonidine suppression test may eliminate the source of
sympathetic nervous system false-positive results, but it is rarely used in
clinical practice. Therefore, one must often rely on clinical judgment and
close monitoring to assess for progressive symptoms.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-50-320.jpg)


![ITE 2018 Question 83
Laboratory test results:
• Sodium = 138 mEq/L (136-142 mEq/L) (SI: 138 mmol/L [136-142 mmol/L])
• Potassium = 4.9 mEq/L (3.5-5.0 mEq/L) (SI: 4.9 mmol/L [3.5-5.0 mmol/L])
• Serum urea nitrogen = 18 mg/dL (8-23 mg/dL) (SI: 6.4 mmol/L [2.9-8.2 mmol/L])
• Creatinine = 0.9 mg/dL (0.7-1.3 mg/dL) (SI: 79.6 μmol/L [61.9-114.9 μmol/L])
• Glucose = 175 mg/dL (70-99 mg/dL) (SI: 9.7 mmol/L [3.9-5.5 mmol/L])
• TSH = 2.4 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 1.3 ng/dL (0.8-1.8 ng/dL) (SI: 16.7 pmol/L [10.30-23.17 pmol/L])
• Cortisol (8 AM, after 1-mg overnight dexamethasone suppression test) = 14
μg/dL (SI: 386.2 nmol/L)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-54-320.jpg)



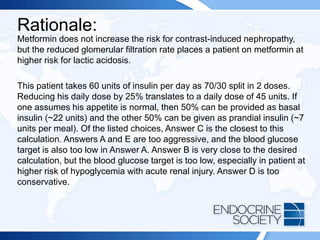
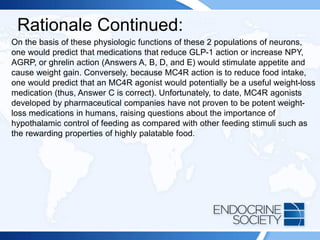
![Rationale Continued:
This patient is taking carbamazepine, which is known to induce hepatic
CYP3A4 enzymes that increase the metabolism of dexamethasone and
potentially lead to a false-positive result. Therefore, any further screening
test that uses dexamethasone (eg, low-dose dexamethasone suppression
test [Answer D]) would be similarly affected. Measuring plasma
dexamethasone at the time of the dexamethasone suppression test is
helpful in identifying inadequate exposure to dexamethasone during
testing. Other inducers of hepatic CYP3A4 that can cause false-positive
results in this setting include mitotane, rifampicin, barbiturates, and
phenytoin. Lamotrigine does not affect dexamethasone metabolism in this
way, so repeating the test after stopping lamotrigine (Answer E) would not
be helpful. Moreover, it is inadvisable to stop an anticonvulsant medication
without consulting the patient’s neurologist.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-58-320.jpg)



![ITE 2018 Question 86
• Sodium = 143 mEq/L (136-142 mEq/L) (SI: 143 mmol/L [136-142 mmol/L])
• Potassium = 3.1 mEq/L (3.5-5.0 mEq/L) (SI: 3.1 mmol/L [3.5-5.0 mmol/L])
• Serum aldosterone = <2 ng/dL (1-21 ng/dL) (SI: <55.5 pmol/L [27.7-582.5
pmol/L])
• Plasma renin activity = <0.6 ng/mL per h (0.6-4.3 ng/mL per h)
• Plasma ACTH = 11 pg/mL (10-60 pg/mL) (SI: 2.4 pmol/L [2.2-13.2 pmol/L])
• Serum cortisol = 14 µg/dL (5-25 µg/dL) (SI: 386.2 nmol/L [137.9-689.7 nmol/L])
• Serum DHEA-S = 2833 μg/dL (18-244 μg/dL) (SI: 76.8 μmol/L [0.49-6.61
μmol/L])
• Serum total testosterone = 310 ng/dL (8-60 ng/dL) (SI: 10.8 nmol/L [0.3-2.1
nmol/L])
• Sex hormone–binding globulin = 1.0 µg/mL (2.2-14.6 µg/mL) (SI: 8.9 nmol/L [20-
130 nmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-62-320.jpg)





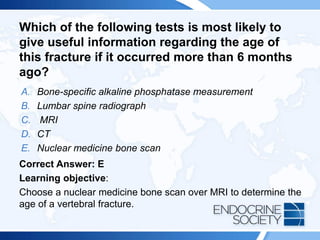
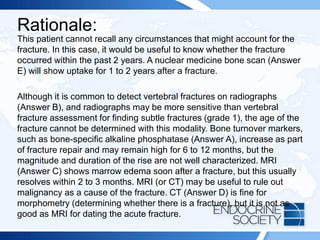


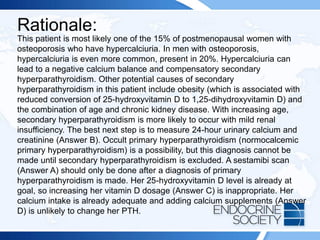
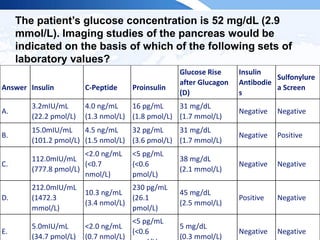
![ITE 2018 Question 8
You are asked to see a 64-year-old woman who was found to have an elevated
serum PTH level of 87 pg/mL (10-65 pg/mL) (SI: 87 ng/L [10-65 ng/L]) as part of an
evaluation for low bone mass (lowest T scores, –2.1 in the spine and –1.6 in the left
femoral neck) documented on routine DXA testing. Her BMI is 32 kg/m2. She has
been taking 2000 IU of vitamin D daily for several years, and her daily calcium intake
is from a balanced diet that includes 3 cups of milk per day without calcium
supplements. Her serum calcium levels have consistently ranged between 9.3 and
10.0 mg/dL (2.3-2.5 mmol/L), within the laboratory’s reference range.
Other laboratory test results:
• Serum 25-hydroxyvitamin D = 48 ng/mL (25-80 ng/mL [optimal]) (SI: 119.8
nmol/L [62.4-199.7 nmol/L])
• Serum creatinine = 1.1 mg/dL (0.6-1.1 mg/dL) (SI: 97.2 µmol/L [53.0-97.2
µmol/L])
• Repeated PTH = 79 pg/mL (10-65 pg/mL) (SI: 79 ng/L [10-65 ng/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-73-320.jpg)


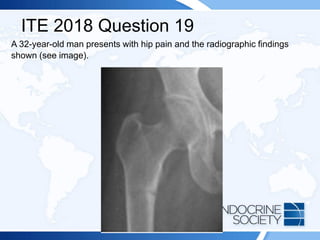
![ITE 2018 Question 19
Laboratory test results:
• Serum calcium = 8.2 mg/dL (8.2-10.2 mg/dL) (SI: 2.1 mmol/L [2.1-2.6
mmol/L])
• Phosphate = 2.2 mg/dL (2.3-4.7 mg/dL) (SI: 0.7 mmol/L [0.7-1.5
mmol/L])
• Creatinine = 0.9 mg/dL (0.7-1.3 mg/dL) (SI: 79.6 µmol/L [61.9-114.9
µmol/L])
• Serum alkaline phosphatase = 346 U/L (50-120 U/L) (SI: 5.78 µkat/L
[0.84-2.00 µkat/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-78-320.jpg)

![Rationale:
Shown on the radiograph is a Looser zone, characteristic of osteomalacia.
Mechanical stress of blood vessels overlying the uncalcified cortical bone affected
by osteomalacia is thought to cause “pseudo fractures” that appear as transverse
zones of rarefaction, sometimes as wide as 1 cm, often multiple, and generally
symmetric. Typical locations are the ischium, ilium, pubis, femur, tibia, radius,
fibula, lower ribs, and scapula.
This patient has a longstanding history of celiac disease and nonadherence to
dietary recommendations. His serum 25-hydroxyvitamin D level (Answer C) was
undetectable (<7 ng/mL [<17.5 nmol/L]). High-dosage vitamin D3, 100,000 IU
daily, did not correct the vitamin D deficiency, but ultraviolet light (tanning salon)
was successful. Chemical clues to osteomalacia include hypocalcemia,
hypophosphatemia, and elevated alkaline phosphatase. Excess fibroblast growth
factor 23 (Answer A) causes hypophosphatemia, but it is rare. While a low serum
1,25-dihydroxyvitamin D level (Answer B) would produce this pattern, it is usually
caused by vitamin D–dependent rickets type 1 (1α-hydroxylase deficiency) and is
typically diagnosed in childhood. Although this patient may have a high PTH level
(Answer D), its measurement would not identify the underlying cause.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-80-320.jpg)

![ITE 2018 Question 38
A 68-year-old man is admitted to the hospital for worsening back pain and
obstructive urinary symptoms. His only home medication is
hydrochlorothiazide, 50 mg daily. CT shows a large necrotic prostate mass
with metastases to the pelvis and spine. Laboratory test results reveal a
calcium concentration of 15.1 mg/dL (8.2-10.2 mg/dL) (SI: 3.8 mmol/L [2.1-
2.6 mmol/L]), and he is given 4 mg of intravenous zoledronic acid.
Additionally, high-dosage dexamethasone is initiated for spinal cord
compromise, and high-dosage ketoconazole is initiated for rapid medical
castration.
You are now consulted for symptomatic hypocalcemia that has been present
for 4 days since his admission despite frequent infusions of calcium
gluconate, 1 g intravenously. When you tap the skin over his facial nerve,
contractions are seen. His reflexes are brisk.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-82-320.jpg)
![ITE 2018 Question 38
Laboratory test results:
• Serum total calcium = 5.6 mg/dL (8.2-10.2 mg/dL) (SI: 1.4 mmol/L [2.1-2.6 mmol/L])
• Ionized calcium = 2.9 mg/dL (4.60-5.08 mg/dL) (SI: 0.7 mmol/L [1.2-1.3 mmol/L])
• Phosphate = 1.7 mg/dL (2.3-4.7 mg/dL) (SI: 0.5 mmol/L [0.7-1.5 mmol/L])
• Serum creatinine = 1.3 mg/dL (0.7-1.3 mg/dL) (SI: 114.9 µmol/L [61.9-114.9
µmol/L])
• Alkaline phosphatase = 81 U/L (50-120 U/L) (SI: 1.35 µkat/L [0.84-2.00 µkat/L])
• PTH = 245 pg/mL (10-65 pg/mL) (SI: 245 ng/L [10-65 ng/L])
• PTHrP = 34.9 pg/mL (14-27 pg/mL) (SI: 34.9 ng/L [14-27 ng/L])
• Magnesium = 2.0 mg/dL (1.5-2.3 mg/dL) (SI: 0.8 mmol/L [0.6-0.9 mmol/L])
• Albumin = 2.9 g/dL (3.5-5.0 g/dL) (SI: 29 g/L [35-50 g/L])
• 25-Hydroxyvitamin D = <8 ng/mL (25-80 ng/mL [optimal]) (SI: <20.0 nmol/L [62.4-
199.7 nmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-83-320.jpg)




![ITE 2018 Question 40
After passing a kidney stone, a generally healthy 20-year-old woman is referred to
you by her urologist. She does not take any calcium or vitamin supplements.
Laboratory test results:
• Serum calcium = 11.0 mg/dL (8.2-10.2 mg/dL) (SI: 2.8 mmol/L [2.1-2.6 mmol/L])
• 25-Hydroxyvitamin D = 70 ng/mL (25-80 ng/mL [optimal]) (SI: 174.7 nmol/L [62.4-
199.7 nmol/L])
• 1,25-Dihydroxyvitamin D = 75 pg/mL (16-65 pg/mL) (SI: 195 pmol/L [41.6-169.0
pmol/L])
• PTH = <10 pg/mL (10-65 pg/mL) (SI: <10 ng/L [10-65 ng/L])
• Serum protein electrophoresis and urine protein electrophoresis, normal
• PTHrP, undetectable
A PET-CT is normal. Her brother also has nephrolithiasis.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-89-320.jpg)



![ITE 2018 Question 42
Laboratory test results:
• Alanine aminotransferase, normal
• Aspartate aminotransferase, normal
• Complete blood count, normal
• Alkaline phosphatase = 412 U/L (50-120 U/L) (SI: 6.88 mkat/L [0.84-2.00 mkat/L])
• 25-Hydroxyvitamin D = 25 ng/mL (25-80 ng/mL [optimal]) (SI: 62.4 nmol/L [62.4-199.7 nmol/L])
• 1,25-Dihydroxyvitamin D = 52.6 pg/mL (16-65 pg/mL) (SI: 136.8 pmol/L [41.6-169.0 pmol/L])
• PTH = 65 pg/mL (10-65 pg/mL) (SI: 65 ng/L [10-65 ng/L])
• Creatinine = 0.9 mg/dL (0.6-1.1 mg/dL) (SI: 79.6 mmol/L [53.0-97.2 mmol/L])
• Calcium = 8.9 mg/dL (8.2-10.2 mg/dL) (SI: 2.2 mmol/L [2.1-2.6 mmol/L])
• Phosphate = 1.2 mg/dL (2.3-4.7 mg/dL) (SI: 0.4 mmol/L [0.7-1.5 mmol/L])
• Fibroblast growth factor 23 = 150 RU/mL (<180 RU/mL)
• Urinalysis dipstick, normal
• Tubular reabsorption of phosphate (TRP) = 80% (>95%)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-94-320.jpg)



![ITE 2018 Question 50
A 38-year-old man is referred to you for persistent hypercalcemia. He has no history
of peptic ulcer disease, nephrolithiasis, or hypertension. Eight months ago, he
underwent parathyroidectomy on the basis of the following laboratory values:
• Serum calcium = 11.5 mg/dL (8.2-10.2 mg/dL) (SI: 2.88 mmol/L [2.1-2.6 mmol/L])
• PTH = 50 pg/mL (10-65 pg/mL) (SI: 50 ng/L [10-65 ng/L])
• Serum creatinine = 1.05 mg/dL (0.7-1.3 mg/dL) (SI: 92.8 mmol/L [61.9-114.9
µmol/L])
• 25-Hydroxyvitamin D = 22 ng/mL (25-80 ng/mL [optimal]) (SI: 54.9 nmol/L [62.4-
199.7 nmol/L])
• Serum phosphate = 2.1 mg/dL (2.3-4.7 mg/dL) (SI: 0.68 mmol/L [0.7-1.5 mmol/L])
Two enlarged glands were resected during the operation, with the final pathology
report documenting hyperplasia in both glands. However, intraoperative PTH levels
remained elevated. Postoperatively, his calcium concentration was 11.6 mg/dL (2.90
mmol/L) and his PTH concentration was 54 pg/mL (54 ng/L).](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-99-320.jpg)
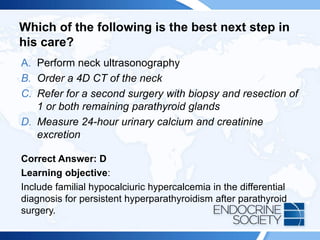
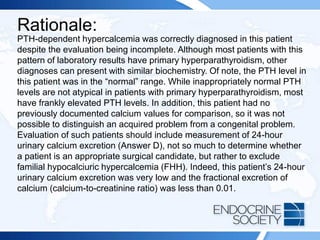
![Rationale Continued:
Calculating a calcium-to-creatinine clearance ratio is helpful to distinguish
FHH from primary hyperparathyroidism, as 80% of patients with FHH
have a ratio less than 0.01 and most patients with primary
hyperparathyroidism have a ratio greater than 0.02. The calcium-to-
creatinine clearance is calculated with the following equation: [24 h
urinary calcium X serum creatinine] ¸ [serum calcium X 24 h urinary
creatinine].
FHH is an autosomal dominant disorder with high penetrance and is
caused by inactivating mutations in the CASR gene. More than 200
CASR mutations have been described. In this disorder, the parathyroid
glands have reduced sensitivity to calcium and higher serum calcium
levels are needed to reduce parathyroid release. The calcium-PTH curve
is shifted to the right, resetting the serum calcium to a higher level. In the
kidney, this produces an increase in tubular calcium and magnesium
reabsorption.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-102-320.jpg)


![ITE 2018 Question 58
You are called by an emergency department physician for advice in caring for a
35-year-old woman with a 5-year history of postsurgical hypoparathyroidism,
previously well controlled, who has come to the emergency department after
having a seizure. She is now conscious but confused. She has been out of town
and without her medication for 3 days. Yesterday, she told her daughter she was
having some problems with tingling and muscle spasms.
Laboratory test results:
• Calcium = 5.8 mg/dL (8.2-10.2 mg/dL) (SI: 1.5 mmol/L [2.1-2.6 mmol/L])
• Albumin = 3.8 g/dL (3.5-5.0 g/dL) (SI: 38 g/L [3.5-5.0 g/L])
• Phosphate = 5.3 mg/dL (2.3-4.7 mg/dL) (SI: 1.7 mmol/L [0.7-1.5 mmol/L])
• Magnesium = 1.9 mg/dL (1.5-2.3 mg/dL) (SI: 0.78 mmol/L [0.6-0.9 mmol/L])
• Creatinine = 0.9 mg/dL (0.6-1.1 mg/dL) (SI: 79.6 µmol/L [53.0-97.2 µmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-106-320.jpg)



![ITE 2018 Question 63
An 80-year-old woman with Paget disease is referred to you for evaluation.
Ten years ago, a nuclear bone scan showed increased uptake limited to the
right tibia, and radiographs showed pagetic changes of the tibia. Her alkaline
phosphatase level at that time was 125 U/L (2.09 µkat/L) (reference range,
50-120 U/L [0.84-2.00 µkat/L]). She has not received treatment. She now
describes severe pain in her right tibia and a radiograph shows coarsened
trabeculae. Her alkaline phosphatase concentration is 250 U/L (4.18 µkat/L).
Her gamma-glutamyltranspeptidase level is normal.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-110-320.jpg)



![ITE 2018 Question 69
You are asked to see a 38-year-old man in the emergency department after he had a
seizure and was found to be hypocalcemic. His height is 70 in (177.8 cm), and he
has no history of calcium or bone problems. In querying about his family history, you
learn that his mother died at age 25 years in a car crash that occurred after she
experienced a seizure (she was reportedly otherwise healthy). The patient has 2
healthy teenaged children.
Laboratory test results:
• Serum calcium = 6.5 mg/dL (8.2-10.2 mg/dL) (SI: 1.6 mmol/L [2.1-2.6 mmol/L])
• Albumin = 3.8 g/dL (3.5-5.0 g/dL) (SI: 38 g/L [3.5-5.0 g/L])
• Serum phosphate = 5.6 mg/dL (2.3-4.7 mg/dL) (SI: 1.8 mmol/L [0.7-1.5 mmol/L])
• Serum creatinine = 0.8 mg/dL (0.7-1.3 mg/dL) (SI: 70.7 µmol/L [61.9-114.9
µmol/L])
• PTH = 5 pg/mL (10-65 pg/mL) (SI: 5 ng/L [10-65 ng/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-114-320.jpg)
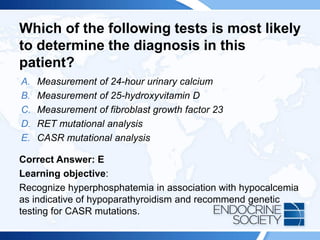
![Rationale:
The patient’s elevated serum phosphate tells you that the cause of his
hypocalcemia is hypoparathyroidism, not vitamin D deficiency. In approximately
one-half of patients with hypoparathyroidism, the cause is an activating CASR
mutation (Answer E). Other known causes of hypoparathyroidism include
autoimmune disease (eg, polyglandular failure due to APECED [autoimmune
polyendocrinopathy-candidiasis-ectodermal dystrophy]), infiltrative disease (eg,
hemochromatosis), HIV infection, and truly idiopathic hypoparathyroidism.
Hypoparathyroidism due to CASR mutations is inherited in an autosomal
dominant manner, so this patient’s mother was most likely also affected. If a
mutation is found in this patient, the family should be counseled about
presymptomatic testing for his children.
Fibroblast growth factor 23 excess (Answer C) causes hypophosphatemia, not
hyperphosphatemia. Measuring 24-hour urinary calcium excretion (Answer A) will
not help make the diagnosis. If this were vitamin D deficiency (Answer B), the
patient’s serum phosphate level would be low or normal, not elevated. Mutations
in the RET proto-oncogene (Answer D) are associated with multiple endocrine
neoplasia type 2 and genetic testing will not help in this patient’s assessment.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-116-320.jpg)


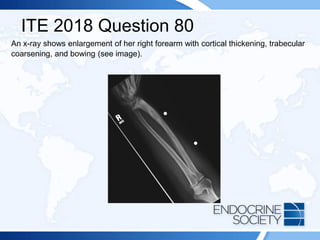
![ITE 2018 Question 80
She describes paresthesias in her right fingers, as well as pain that extends up to her
right shoulder.
She has no axillary lymphadenopathy. Her right upper extremity has an obvious
distal radial deformity with an apex dorsal bow and is tender to palpation but is
neurovascularly intact with no sensory deficits.
Laboratory test results:
• Serum total calcium = 9.4 mg/dL (8.2-10.2 mg/dL) (SI: 2.4 mmol/L [2.1-2.6 mmol/L])
• Phosphate = 3.7 mg/dL (2.3-4.7 mg/dL) (SI: 1.2 mg/dL [0.7-1.5 mmol/L])
• Serum creatinine = 0.7 mg/dL (0.6-1.1 mg/dL) (SI: 61.9 µmol/L [53.0-97.2 µmol/L])
• Alkaline phosphatase = 381 U/L (50-120 U/L) (SI: 6.4 µkat/L [0.84-2.00 µkat/L])
• PTH = 47 pg/mL (10-65 pg/mL) (SI: 47 ng/L [10-65 ng/L])
• Urinary N-telopeptide = 189 nmol BCE/mmol creat (24-124 nmol BCE/mmol creat)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-120-320.jpg)





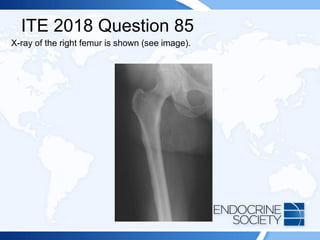
![ITE 2018 Question 85
Laboratory test results:
• Calcium = 8.8 mg/dL (8.2-10.2 mg/dL) (SI: 2.2 mmol/L [2.1-2.6 mmol/L])
• Phosphate = 3.0 mg/dL (2.3-4.7 mg/dL) (SI: 1.0 mmol/L [0.7-1.5 mmol/L])
• Albumin = 3.8 g/dL (3.5-5.0 g/dL) (SI: 38 g/L [35-50 g/L])
• Alkaline phosphatase = 135 U/L (50-120 U/L) (SI: 2.3 μkat/L [0.84-2.00 μkat/L])
• 25-Hydroxyvitamin D = 19 ng/mL (25-80 ng/mL [optimal]) (SI: 47.4 nmol/L [62.4-
199.7 nmol/L])
• 1,25-Dihydroxyvitamin D = 30 pg/mL (16-65 pg/mL) (SI: 78 pmol/L [41.6-169.0
pmol/L])
• Intact PTH = 63 pg/mL (10-65 pg/mL) (SI: 63 ng/L [10-65 ng/L])
• Osteocalcin = 21.9 ng/mL (9.0-42.0 ng/mL) (SI: 21.9 µg/L [9.0-42.0 µg/L])
• Serum C-telopeptide = 93 pg/mL (104-1008 pg/mL [postmenopausal women])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-127-320.jpg)





![ITE 2018 Question 1
On physical examination, his height is 70 in (177.8 cm) and weight is 200 lb
(90.9 kg) (BMI = 28.7 kg/m2).
Laboratory test results on admission:
• Hemoglobin A1c = 8.0% (4.0%-5.6%) (64 mmol/mol [20-38 mmol/mol])
• Estimated glomerular filtration rate = 30 mL/min per 1.73 m2 (>60
mL/min per 1.72 m2)
• Glucose = 120 mg/dL (70-99 mg/dL) (SI: 6.7 mmol/L [3.9-5.5 mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-136-320.jpg)
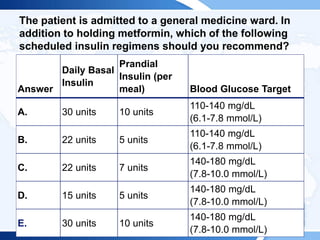





![ITE 2018 Question 10
Physical examination findings are unremarkable.
Laboratory test results:
• Hemoglobin A1c = 7.6% (4.0%-5.6%) (60 mmol/mol [20-38 mmol/mol])
• LDL cholesterol = 52 mg/dL (<100 mg/dL [optimal]) (SI: 1.35 mmol/L
[<2.59 mmol/L])
• Urinary albumin-to-creatinine ratio = 7 mg/g creat (<30 mg/g creat)
The patient asks whether insulin pump therapy would assist with her
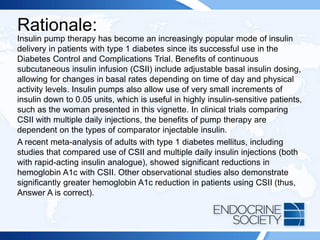
diabetes control.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-144-320.jpg)



![ITE 2018 Question 17
An otherwise healthy 20-year-old African American man comes to clinic for a
follow-up visit. He was hospitalized for treatment of diabetic ketoacidosis 4
months ago.
Laboratory test results at hospital admission:
• Plasma glucose = 748 mg/dL (70-99 mg/dL) (SI: 41.5 mmol/L [3.9-5.5
mmol/L])
• Bicarbonate = 10 mEq/L (21-28 mEq/L) (SI: 10 mmol/L [21-28 mEq/L])
• Anion gap = 22 mEq/L (3-11 mEq/L)
• Creatinine = 2.2 mg/dL (0.7-1.3 mg/dL) (SI: 194.5 µmol/L [61.9-114.9
µmol/L])
• Estimated glomerular filtration rate = 34 mL/min per 1.73 m2 (>60
mL/min per 1.73 m2)
• Moderate ketones present in the serum](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-149-320.jpg)

![ITE 2018 Question 17
On physical examination, his height is 73 in (185 cm) and weight is 242 lb (110
kg) (BMI = 31.9 kg/m2). His blood pressure is 122/83 mm Hg, and pulse rate is
82 beats/min. He has central weight distribution. There is evidence of
acanthosis nigricans. The rest of the examination findings are normal.
Current laboratory test results (fasting):
• Hemoglobin A1c = 5.8% (4.0%-5.6%) (40 mmol/mol [20-38 mmol/mol])
• Creatinine = 1.3 mg/dL (0.7-1.3 mg/dL) (SI: 114.9 µmol/L [61.9-114.9 µmol/L])
• Estimated glomerular filtration rate = >60 mL/min per 1.73 m2 (>60 mL/min per 1.73 m2)
• Electrolytes, normal
• TSH, normal
• C-peptide = 3.2 ng/mL (0.9-4.3 ng/mL) (SI: 1.06 nmol/L [0.30-1.42 nmol/L])
• Glucose = 124 mg/dL (70-99 mg/dL) (SI: 6.9 mmol/L [3.9-5.5 mmol/L])
• Glutamic acid decarboxylase antibodies, undetectable](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-151-320.jpg)








![ITE 2018 Question 23
Laboratory test results:
• Hemoglobin A1c = 8.0% (4.0%-5.6%) (64 mmol/mol [20-38 mmol/mol])
• Creatinine = 1.3 mg/dL (0.7-1.3 mg/dL) (SI: 114.9 µmol/L [61.9-114.9 µmol/L])
• Estimated glomerular filtration rate = 60 mL/min per 1.73 m2 (>60 mL/min per
1.73 m2)
• Electrolytes, normal
• TSH, normal
• Vitamin B12 and folic acid, normal
• Creatine kinase, normal
• Serum electrophoresis, no monoclonal protein
• Erythrocyte sedimentation rate = 24 mm/h (0-20 mm/h)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-161-320.jpg)





![Reference(s):
• Barohn RJ, Sahenk Z, Warmolts JR, Mendell JR. The Bruns-Garland syndrome
(diabetic amyotrophy). Revisited 100 years later. Arch Neurol. 1991;48(11):1130-1135.
PMID: 1953396
• Bastron JA, Thomas JE. Diabetic polyradiculopathy: clinical and electromyographic
findings in 105 patients. Mayo Clin Proc. 1981;56(12):725-732. PMID: 7311600
• Dyck PJ, Norell JE, Dyck PJ. Microvasculitis and ischemia in diabetic lumbosacral
radiculoplexus neuropathy. Neurology. 1999;53(9):2113-2121. PMID: 10599791
• Zochodne DW, Isaac D, Jones C. Failure of immunotherapy to prevent, arrest or
reverse diabetic lumbosacral plexopathy. Acta Neurol Scand. 2003;107(4):299-301.
PMID: 12675705
• Van den Bergh PY, Hadden RD, Bouche P, et al; European Federation of Neurological
Societies; Peripheral Nerve Society. European Federation of Neurological
Societies/Peripheral Nerve Society guideline on management of chronic inflammatory
demyelinating polyradiculopathy: report of a joint task force of the European
Federation of Neurological Societies and the Peripheral Nerve Society - first revision
[published correction appears in Eur J Neurol. 2011;18(5):796]. Eur J Neurol.
2010;17(3):356-363. PMID: 20456730
• Thaisetthawatkul P, Dyck PJ. Treatment of diabetic and nondiabetic lumbosacral
radiculoplexus neuropathy. Curr Treat Options Neurol. 2010;12(2):95-99. PMID:
20842573](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-167-320.jpg)








![ITE 2018 Question 33
Morning Lunch Dinner Bedtime Overnight Overall
Mean
glucose
136 mg/dL
(±20)
(7.6 mmol/L
[±1.1])
142 mg/dL
(±34) (7.9
mmol/L
[±1.9])
133 mg/dL
(±27)
(7.4 mmol/L
[±1.5])
132 mg/dL
(±31)
(7.3 mmol/L
[±2.3])
96 mg/dL
(±19)
(5.3 mmol/L
[±1.1])
128 mg/dL
(±21)
(7.1 mmol/L
[±1.2])
Values are presented with standard deviation in parentheses.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-177-320.jpg)



![Reference(s):
• Kahn R, Fonseca V. Translating the A1C assay. Diabetes Care.
2008;31(8):1704-1707. PMID: 18540045
• Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ;
A1c-Derived Average Glucose Study Group. Translating the A1C
assay into estimated average glucose values [published correction
appears in Diabetes Care. 2009;32(1):207]. Diabetes Care.
2008;31(8):1473-1478. PMID: 18540046
• Wiwanitkit V. Problem of using hemoglobin A1C measurement in
endemic area of hemoglobinopathy. Prim Care Diabetes.
2007;1(3):173-175. PMID: 18632040](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-181-320.jpg)











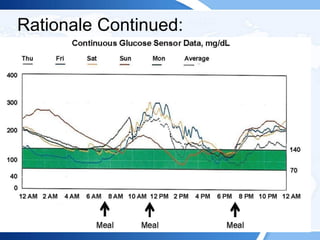


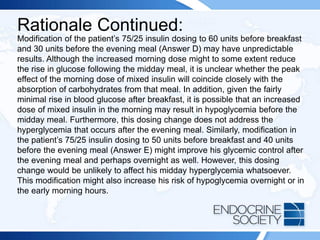







![ITE 2018 Question 59
On physical examination, her height is 66 in (168 cm) and weight is 136 lb
(61.8 kg) (BMI = 21.9 kg/m2). Her blood pressure is 106/72 mm Hg, and
pulse rate is 62 beats/min. Examination findings are normal.
Laboratory test results:
• Hemoglobin A1c = 5.9% (4.0%-5.6%) (41 mmol/mol [20-38 mmol/mol])
• Creatinine = 0.7 mg/dL (0.6-1.1 mg/dL) (SI: 61.9 µmol/L [53.0-97.2 µmol/L])
• Estimated glomerular filtration rate = >90 mL/min per 1.73 m2 (>60 mL/min per
1.73 m2)
• Electrolytes, normal
• TSH, normal
• C-peptide = 1.8 ng/mL (0.9-4.3 ng/mL) (SI: 0.6 nmol/L [0.30-1.42 nmol/L])
• Glucose = 136 mg/dL (70-99 mg/dL) (SI: 7.5 mmol/L [3.9-5.5 mmol/L])
• Glutamic acid decarboxylase antibodies, undetectable](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-208-320.jpg)




![ITE 2018 Question 62
Recent laboratory test results:
• Hemoglobin A1c = 6.8% (4.0%-5.6%) (51 mmol/mol [20-38 mmol/mol])
• Fasting plasma glucose = 94 mg/dL (70-99 mg/dL) (SI: 5.2 mmol/L [3.9-
5.5 mmol/L])
• Total cholesterol = 189 mg/dL (<200 mg/dL [optimal]) (SI: 4.90 mmol/L
[<5.18 mmol/L])
• Triglycerides = 120 mg/dL (<150 mg/dL [optimal]) (SI: 1.36 mmol/L
[<1.70 mmol/L])
• LDL cholesterol = 135 mg/dL (<100 mg/dL [optimal]) (SI: 3.50 mmol/L
[<2.59 mmol/L])
• HDL cholesterol = 40 mg/dL (>60 mg/dL [optimal]) (SI: 1.04 mmol/L
[>1.55 mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-215-320.jpg)

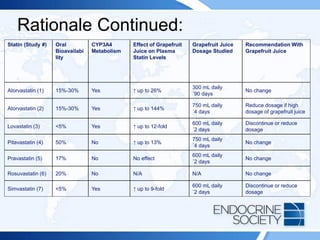
![Rationale:
Current recommendations for statin treatment have been revised such that
treatment initiation and the initial statin dosage are personalized on the basis
of risk profile, rather than LDL-cholesterol levels. In patients with type 2
diabetes who are 40 years or older, moderate-intensity statin treatment, if
clinically indicated, is recommended in addition to lifestyle counseling and
behavioral modification. However, for patients with a high risk for
cardiovascular disease (defined as an LDL-cholesterol level of 100 mg/dL or
greater [≥2.6 mmol/L], high blood pressure, history of cigarette smoking,
overweight/obesity, or a history of cardiovascular disease), high-intensity
statin therapy is advised. Clinical trials have shown that individuals at high
risk for cardiovascular disease have a significant reduction in further
cardiovascular events with an aggressive regimen of high-intensity statin
therapy. Currently, limited clinical trial evidence is available for statin therapy
for persons older than 75 years or younger than 40 years. The only high-
intensity statin therapy listed in the answer options is rosuvastatin, 20 mg
daily (Answer B). Answers A, C, and D are all moderate-intensity statin
therapy options.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-217-320.jpg)
![Reference(s):
• Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA
guideline on the treatment of blood cholesterol to reduce
atherosclerotic cardiovascular risk in adults: a report of the American
College of Cardiology/American Heart Association Task Force on
Practice Guidelines [published corrections appear in J Am Coll
Cardiol. 2014;63(25 Pt B):3024-3025 and J Am Coll Cardiol.
2015;66(24):2812]. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934.
PMID: 24239923
• American Diabetes Association. Standards of medical care in
diabetes--2017. Diabetes Care. 2017;40(Suppl 1):S1-S135.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-218-320.jpg)

![ITE 2018 Question 68
Laboratory test results:
• Electrolytes and creatinine, normal
• Glucose (fasting) = 168 mg/dL (70-99 mg/dL) (SI: 9.3 mmol/L [3.9-5.5 mmol/L])
• Insulin (fasting) = 43 µIU/mL (1.4-14.0 µIU/mL) (SI: 298.6 pmol/L [9.7-97.2 pmol/L])
• Triglycerides (fasting) = 350 mg/dL (<150 mg/dL [optimal]) (3.96 mmol/L [<1.70 mmol/L])
• Hemoglobin A1c = 6.2% (4.0%-5.6%) (44 mmol/mol [20-38 mmol/mol])
• Plasma ACTH (8 AM) = 22 pg/mL (10-60 pg/mL) (SI: 4.8 pmol/L [2.2-13.2 pmol/L])
• Urinary free cortisol = 13 µg/24 h (4-50 µg/24 h) (SI: 35.9 nmol/d [11-138 nmol/d])
(creatinine = 1.2 g)
• Serum cortisol (8 AM) after 1 mg dexamethasone at 11 PM the previous night = 0.9 µg/dL
(SI: 24.8 nmol/L)
• Serum testosterone = 40 ng/dL (8-60 ng/dL) (SI: 1.4 nmol/L [0.3-2.1 nmol/L])
• Serum sex hormone–binding globulin = 1.3 µg/mL (2.2-14.6 µg/mL) (SI: 12 nmol/L [20-130
nmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-220-320.jpg)
![Which of the following is the best test to
establish the diagnosis?
A. [111In]-pentetreotide (octreotide) scan
B. CT of the adrenal glands
C. MRI of the liver
D. MRI of the sella
E. MRI of the legs
Correct Answer: E
Learning objective:
Recognize and diagnose lipodystrophy.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-221-320.jpg)







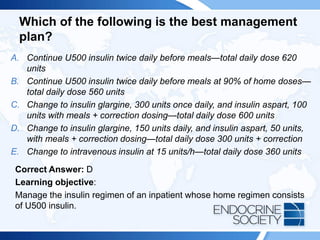



![Rationale Continued:
The group maintained on U500 insulin had more days with hypoglycemia but
a shorter length of stay than the group converted to U100 insulin, and the
authors concluded that use of U500 insulin during admission may be
appropriate for some patients. Another single-center retrospective study
compared inpatient insulin doses with home insulin doses for U500 insulin
users (all patients’ regimens were converted to U100 insulin during
admission) and reported that the average inpatient insulin dose was only
22.6% of the usual home dose. Patients were stratified into 3 groups based
on their preadmission hemoglobin A1c level (<8%, 8%-9%, >9% [<64, 64-75,
>75 mmol/mol]). All 3 groups had dose reductions of 75% to 80% compared
with the home insulin dose, yet hypoglycemia was present in 2% to 4% of
point-of-care blood glucose readings. The group with the highest
preadmission hemoglobin A1c value had the most hyperglycemia during
inpatient admissions. A notable difference between the 2 studies is the home
total daily insulin use: the first study (Tripathy et al) had home insulin doses
of 200 U100 units daily, whereas the second study (Palladino et al) reported
home insulin doses of 300 to 500 U100 units daily.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-236-320.jpg)






![ITE 2018 Question 74
Laboratory test results:
• Hemoglobin A1c = 8.3% (4.0%-5.6%) (67 mmol/mol [20-38 mmol/mol])
• Fasting glucose = 142 mg/dL (70-99 mg/dL) (SI: 7.9 mmol/L [3.9-5.5
mmol/L])
• Serum urea nitrogen = 31 mg/dL (8-23 mg/dL) (SI: 11.1 mmol/L [2.9-8.2
mmol/L])
• Creatinine = 1.8 mg/dL (0.7-1.3 mg/dL) (SI: 159.1 µmol/L [61.9-114.9
µmol/L])
• Estimated glomerular filtration rate = 40 mL/min per 1.73 m2 (>60
mL/min per 1.73 m2)
• Liver function, normal](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-245-320.jpg)




![ITE 2018 Question 77
Physical examination findings are normal.
Laboratory test results:
• Hemoglobin A1c = 7.1% (4.0%-5.6%) (54 mmol/mol [20-38 mmol/mol])
• Creatinine = 1.0 mg/dL (0.7-1.3 mg/dL) (SI: 88.4 µmol/L [61.9-114.9
µmol/L])
• Estimated glomerular filtration rate = >90 mL/min per 1.73 m2 (>60
mL/min per 1.73 m2)
• Electrolytes, normal](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-251-320.jpg)






![ITE 2018 Question 78
On physical examination, her blood pressure is 135/78 mm Hg and pulse
rate is 88 beats/min. Her height is 63 in (160 cm), and weight is 317 lb
(144.1 kg) (BMI = 56.1 kg/m2). Her weight is unchanged from that
documented at her last appointment 4 months ago.
Laboratory test results:
• Hemoglobin A1c = 6.1% (4.0%-5.6%) (43 mmol/mol [20-38 mmol/mol])
• Sodium = 141 mEq/L (136-142 mEq/L) (SI: 141 mmol/L [136-142 mmol/L])
• Potassium = 3.8 mEq/L (3.5-5.0 mEq/L) (SI: 3.8 mmol/L [3.5-5.0 mmol/L])
• Creatinine = 0.7 mg/dL (0.6-1.1 mg/dL) (SI: 61.9 µmol/L [53.0-97.2 µmol/L])
• Glomerular filtration rate = 104 mL/min per 1.73 m2 (>60 mL/min per 1.73 m2)
• Fasting glucose = 115 mg/dL (70-99 mg/dL) (SI: 6.38 mmol/L [3.9-5.5 mmol/L)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-258-320.jpg)




![Reference(s):
• Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a
high-risk state for diabetes development. Lancet. 2012;379(9833):2279-
2290. PMID: 22683128
• Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for
the Management of Overweight and Obesity in Adults: A Report of the
American College of Cardiology/American Heart Association Task Force on
Practice Guidelines and The Obesity Society [published correction appears
in J Am Coll Cardiol. 2014;63(25 Pt B):3029-3030]. J Am Coll Cardiol.
2014;63(Pt B):2985-3023. PMID: 24239920
• Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of
Clinical Endocrinologists’ comprehensive diabetes management algorithm
2013 consensus statement—executive summary. Endocr Pract.
2013;19(3):536-557. PMID: 23816937](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-263-320.jpg)



![Reference(s):
• WHO Expert Consultation. Appropriate body-mass index for Asian
populations and its implications for policy and intervention strategies
[published correction appears in Lancet. 2004;363(9412):902]. Lancet.
2004;363(9403):157-163. PMID: 14726171
• American Diabetes Association. Standards of medical care in diabetes--
2017. Diabetes Care. 2017;40(Suppl 1):S1-S135.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-267-320.jpg)

![ITE 2018 Question 88
Laboratory test results (sample drawn while fasting):
• TSH = 1.1 mIU/L (0.5-5.0 mIU/L)
• Glucose = 119 mg/dL (70-99 mg/dL) (SI: 6.6 mmol/L [3.9-5.5 mmol/L])
• Total cholesterol = 224 mg/dL (<200 mg/dL [optimal]) (SI: 5.80 mmol/L
[<5.18 mmol/L])
• Triglycerides = 427 mg/dL (<150 mg/dL [optimal]) (SI: 4.82 mmol/L
[<1.70 mmol/L])
• LDL cholesterol = 92 mg/dL (<100 mg/dL [optimal]) (SI: 2.38 mmol/L
[<2.59 mmol/L])
• HDL cholesterol = 38 mg/dL (>60 mg/dL [optimal]) (SI: 0.98 mmol/L
[>1.55 mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-269-320.jpg)





![ITE 2018 Question 90
Although he has never experienced a driving mishap related to hypoglycemia,
he voluntarily stopped driving when he was referred to you for care. He now
asks whether it would be advisable for him to resume operation of a motor
vehicle for personal use.
On physical examination, the patient is alert and appears well. Blood pressure
is 127/64 mm Hg, and pulse rate is 62 beats/min. His height is 67.5 in (171.5
cm), and weight is 162 lb (73.6 kg) (BMI = 25.0 kg/m2). He has evidence of
previous photocoagulation on undilated funduscopic examination, but visual
fields are grossly intact. He has no foot deformities, foot lesions, or gait
abnormalities. He has a few areas of sensory loss on monofilament testing of
the plantar surfaces of both feet, but proprioception is preserved.
Laboratory test results:
• Hemoglobin A1c = 7.6% (4.0%-5.6%) (60 mmol/mol [20-38 mmol/mol])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-275-320.jpg)







![ITE 2018 Question 22
Laboratory test results (day 5 of an induced menstrual cycle):
• LH = 5.0 mIU/mL (1.0-18.0 mIU/mL [follicular phase]) (SI: 5.0 IU/L [1.0-18.0
IU/L])
• FSH = 4.0 mIU/mL (2.0-12.0 mIU/mL [follicular phase]) (SI: 4.0 IU/L [2.0-12.0
IU/L])
• Estradiol = 40 pg/mL (10-180 pg/mL [follicular phase]) (SI: 146.8 pmol/L [36.7-
660.8 pmol/L])
• Testosterone = 50 ng/dL (8-60 ng/dL) (SI: 1.74 nmol/L [0.3-2.1 nmol/L])
• 17-Hydroxyprogesterone = 330 ng/dL (<285 ng/dL [luteal phase]; <80 ng/dL
[follicular phase]) (SI: 10.0 nmol/L [<8.6 nmol/L] [luteal phase]; [<2.4 nmol/L]
[follicular phase])
• DHEA-S = 440 µg/dL (44-332 µg/dL) (SI: 11.9 µmol/L [1.19-9.00 µmol/L])
• Prolactin = 18 ng/mL (4-30 ng/dL) (SI: 0.78 nmol/L [0.17-1.30 nmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-284-320.jpg)

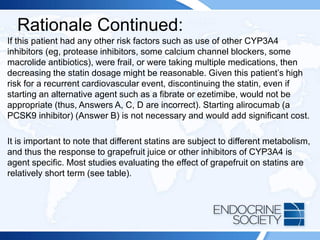
![Rationale Continued:
Cushing syndrome can be associated with adrenal hyperandrogenism,
but the findings on physical examination in this patient do not support that
diagnosis. Moreover, the half-life of DHEA-S is long (days) and dynamic
testing using glucocorticoid suppression (Answer A) must be carried out
for a longer duration (eg, 3-5 days). A patient with polycystic ovary
syndrome could present with a scenario similar to that of this patient, but
the laboratory data of a borderline elevated 17-hydroxyprogesterone
value is more consistent with nonclassic CAH. Thus, proceeding to an
imaging study of the adrenal glands (Answer C) is incorrect. A patient
with a prolactinoma may present with hirsutism and often an elevated
DHEA-S level, but not elevated testosterone or 17-hydroxyprogesterone.
Thus, pituitary MRI (Answer B) is not the best next step. Ovarian tumors
may make a combination of ovarian androgens, but affected patients
usually have a testosterone level in the normal range for a man (240-800
ng/dL [8.3-27.8 nmol/L]), not an elevated 17-hydroxyprogesterone level.
Thus, transvaginal ultrasonography (Answer D) is unnecessary.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-287-320.jpg)
![Reference(s):
• Speiser PW, Azziz R, Baskin LS, et al; Endocrine Society. Congenital
adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an
Endocrine Society Clinical Practice Guideline [published correction
appears in J Clin Endocrinol Metab. 2010;95(11):5137]. J Clin
Endocrinol Metab. 2010;95(9):4133-4160. PMID: 20823466](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-288-320.jpg)

![ITE 2018 Question 36
Laboratory test results from 1 month ago:
• Total testosterone = 69 ng/dL (8-60 ng/dL) (SI: 2.4 nmol/L [0.3-2.1
nmol/L])
• DHEA-S = 115 µg/dL (44-332 µg/dL) (SI: 3.1 µmol/L [1.19-9.00 µmol/L])
• 17-Hydroxyprogesterone = 687 ng/dL (<80 ng/dL [follicular]) (SI: 20.8
nmol/L [<2.4 nmol/L])
• Progesterone = 23.5 ng/mL (≤1.0 ng/mL [follicular]) (SI: 74.7 nmol/L
[≤3.2 nmol/L])
• Estradiol = 1970 pg/mL (10-180 pg/mL [follicular]) (SI: 7232 pmol/L
[36.7-660.8 pmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-290-320.jpg)









![Rationale Continued:
Despite an increase in metabolic syndrome and cardiac risk factors, studies
have not yet shown an increased risk of cardiovascular disease (ie, myocardial
infarction [Answer C] or stroke [Answer B]) in women with polycystic ovary
syndrome. Studies have suggested that women with polycystic ovary
syndrome are at risk for endometrial hyperplasia and endometrial cancer at an
earlier age. In addition, women with polycystic ovary syndrome have a higher
risk of nonalcoholic fatty liver disease, but it is unclear whether treatment
strategies alter this risk. Autoimmune thyroid disease (Answer A) is not
increased in polycystic ovary syndrome. If a patient undergoes ovulation
induction with antiestrogens (clomiphene or letrozole) or gonadotropins, she
will have an increased risk of multiple gestations. Pregnancy complications in
women with polycystic ovary syndrome include gestational diabetes mellitus,
preterm delivery, and preeclampsia. Patients with polycystic ovary syndrome
can have mild hyperprolactinemia, although the medical basis for that finding
is not known. However, they do not have a higher incidence of prolactinoma
(or other types of pituitary tumors) (Answer E) compared with the prevalence in
the general population.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-301-320.jpg)

![ITE 2018 Question 49
A 30-year-old patient (46,XX karyotype) with gender dysphoria presents for
follow-up after initiating hormone treatment. He had undergone psychological
evaluation and lived as a male for the last 6 months before starting androgen
therapy. He was treated with topical testosterone, 50 mg daily. He has never
smoked cigarettes. He is now seen after 6 months for follow-up. He notes
increased facial and body hair, deepening of his voice, and enlargement of the
clitoris. He reports an improvement in strength and an increase in libido.
On physical examination, his blood pressure is 132/70 mm Hg. His height is
66.5 in (168.9 cm), and weight is 197 lb (89.5 kg) (BMI = 31.3 kg/m2). Skin
examination reveals mild acne and increased male- pattern hair over the face,
chest, and extremities with some temporal balding.
Laboratory test results:
Total testosterone = 711 ng/dL (300-900 ng/dL [male]; 8-60 ng/dL [female]) (SI:
24.6 nmol/L [10.4-31.2 nmol/L] [male]; [0.3-2.1 nmol/L] [female])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-303-320.jpg)




![ITE 2018 Question 75
An 18-year-old girl presents with primary amenorrhea and short stature. Her
blood pressure is 140/90 mm Hg. Her height is 56 in (142.2 cm) (BMI = 28
kg/m2). She has absent breast development and scant pubic and axillary hair.
Laboratory test results:
• FSH = 35 mIU/mL (2.0-12.0 mIU/mL [follicular phase]) (SI: 35 IU/L [2.0-
12.0 IU/L])
• LH = 28 mIU/mL (1.0-18.0 mIU/mL [follicular phase]) (SI: 28 IU/L [1.0-18.0
IU/L])
• Estradiol = <10 pg/mL (10-180 pg/mL [follicular phase]) (SI: <36.7 pmol/L
[36.7-660.8 pmol/L])
• Karyotype = 45,X](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-308-320.jpg)








![ITE 2018 Question 82
A 22-year-old woman presents to discuss treatment options for hirsutism. She
had menarche at age 10 years and has always had irregular menses. Acne
and abnormal hair growth began at puberty. She is currently on an oral
contraceptive (ethinyl estradiol 30 mcg/norethindrone 0.5 mg).
On physical examination, her BMI is 27 kg/m2. Excess hair is observed on her
upper lip, chin, and neck. No hair is present on her upper chest, upper back,
or upper abdomen. She has no temporal recession of her hairline. She has
scattered acne. Findings on pelvic examination are normal.
Laboratory test results:
• Testosterone = 75 ng/dL (8-60 ng/dL) (SI: 2.6 nmol/L [0.3-2.1 nmol/L])
• DHEA-S = 297 µg/dL (44-332 µg/dL) (SI: 8.0 µmol/L [1.19-9.00 µmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-320-320.jpg)







![ITE 2018 Question 2
Laboratory test results (fasting):
• Total cholesterol = 200 mg/dL (<200 mg/dL [optimal]) (SI: 5.18 mmol/L
[<5.18 mmol/L])
• LDL cholesterol = 158 mg/dL (<100 mg/dL [optimal]) (SI: 4.09 mmol/L
[<2.59 mmol/L])
• HDL cholesterol = 32 mg/dL (>60 mg/dL [optimal]) (SI: 0.83 mmol/L [>1.55
mmol/L])
• Triglycerides = 176 mg/dL (<150 mg/dL [optimal]) (SI: 1.99 mmol/L [<1.70
mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-328-320.jpg)







![ITE 2018 Question 9
Recent laboratory test results (fasting) while on atorvastatin (but before starting
the grapefruit diet):
• Total cholesterol = 142 mg/dL (<200 mg/dL [optimal]) (SI: 3.68 mmol/L
[<5.18 mmol/L])
• HDL cholesterol = 42 mg/dL (>60 mg/dL [optimal]) (SI: 1.09 mmol/L [>1.55
mmol/L])
• LDL cholesterol = 69 mg/dL (<100 mg/dL [optimal]) (SI: 1.79 mmol/L [<2.59
mmol/L])
• Triglycerides = 98 mg/dL (<150 mg/dL [optimal]) (SI: 1.11 mmol/L [<1.70
mmol/L])
• Creatinine = 0.7 mg/dL (0.6-1.1 mg/dL) (SI: 61.9 µmol/L [53.0-97.2 µmol/L])
• ALT = 28 U/L (10-40 U/L) (SI: 0.47 µkat/L [0.17-0.67 µkat/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-336-320.jpg)







![Reference(s):
• Irizarry KA, Miller M, Freemark M, Haqq AM. Prader Willi syndrome:
genetics, metabolomics, hormonal function, and new approaches to
therapy. Adv Pediatr. 2016;63(1):47-77. PMID: 2742689
• Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M;,
speakers contributors at the Second Expert Meeting of the
Comprehensive Care of Patients with PWS. Recommendations for the
diagnosis and management of Prader-Willi syndrome [published
correction appears in J Clin Endocrinol Metab. 2008;93(11):4183-4197]. J
Clin Endocrinol Metab. 2008;93(11):4183-4197. PMID: 18697869
• Irizarry KA, Bain J, Butler MG, et al. Metabolic profiling in Prader-Willi
syndrome and nonsyndromic obesity: sex differences and the role of
growth hormone. Clin Endocrinol (Oxf). 2015;83(6):797-805. PMID:
25736874
• Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis
of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol
Nutr. 2008;46(1):80-83. PMID: 18162838](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-348-320.jpg)
![ITE 2018 Question 12
You are asked to see a 45-year-old man with a history of type 2 diabetes mellitus for
routine follow-up. He feels well and has no acute concerns. You have been
encouraging his efforts at lifestyle changes with the hope of achieving some weight
loss. His weight of 244 lb (110.9 kg) has been stable over the past year. He is
otherwise healthy. He comments that he was relieved when he recently heard on the
news that lifestyle changes do not really help people with type 2 diabetes, and he
asks whether he must continue his activity program of walking 30 minutes 4 to 5
times per week. He currently takes metformin, 2000 mg daily; simvastatin, 20 mg
daily; and lisinopril, 20 mg daily.
On physical examination, he appears well. His height is 70 in (177.8 cm), and weight
is 244 lb (110.9 kg) (BMI = 35 kg/m2). His blood pressure is 138/80 mm Hg.
Laboratory test results:
• Hemoglobin A1c = 6.8% (4.0%-5.6%) (51 mmol/mol [20-38 mmol/mol])
• Serum creatinine = 1.0 mg/dL (0.7-1.3 mg/dL) (SI: 88.4 mmol/L [61.9-114.9
mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-349-320.jpg)



![Reference(s):
• Look AHEAD Research Group, Wing RR, Bolin P, Brancatti FL, et al.
Cardiovascular effects of intensive lifestyle interventions in type 2
diabetes [published correction appears in N Engl J Med.
2014;370(19):1866]. N Engl J Med. 2013;369(2):145-154. PMID:
23796131](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-353-320.jpg)

















![ITE 2018 Question 65
Laboratory test results (sample drawn while fasting):
• Total cholesterol = 137 mg/dL (<200 mg/dL [optimal]) (SI: 3.55 mmol/L [<5.18
mmol/L])
• Triglycerides = 212 mg/dL (<150 mg/dL [optimal]) (SI: 2.40 mmol/L [<1.70
mmol/L])
• HDL cholesterol = 15 mg/dL (>60 mg/dL [optimal]) (SI: 0.39 mmol/L [>1.55
mmol/L])
• LDL cholesterol = 80 mg/dL (<100 mg/dL [optimal]) (SI: 2.07 mmol/L [<2.59
mmol/L])
• Non-HDL cholesterol = 122 mg/dL (<130 mg/dL [optimal]) (SI: 3.16 mmol/L
[<3.37 mmol/L])
• TSH = 2.1 mIU/L (0.5-5.0 mIU/L)
• Plasma glucose (fasting) = 120 mg/dL (70-99 mg/dL) (SI: 6.7 mmol/L [3.9-5.5
mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-376-320.jpg)

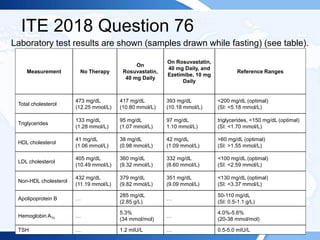
![Rationale:
This patient has a very low HDL-cholesterol level. Low HDL-cholesterol is
currently defined as a value below 40 mg/dL (<1.04 mmol/L) in men or below 50
mg/dL (<1.30 mmol/L) in women, corresponding to approximately the 50th
percentile. Clinical and epidemiologic studies have demonstrated the inverse and
independent association between HDL cholesterol and the risk of coronary heart
disease. Therefore, low HDL cholesterol is established as a classic independent
risk factor and has become part of several multiparametric algorithms used for
cardiovascular risk estimation.
HDL-cholesterol levels below 40 mg/dL (<1.04 mmol/L) are often associated with
hypertriglyceridemia, obesity, insulin resistance, and diabetes. However, marked
HDL-cholesterol deficiency with values below 20 mg/dL (<0.52 mmol/L) is rare
and can be associated very high triglyceride levels (>500 mg/dL [5.65 mmol/L]).
However, in the absence of severe hypertriglyceridemia, such low HDL-
cholesterol levels are associated with perturbations in the HDL metabolic
pathways, which is typically a result of impaired HDL biogenesis.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-378-320.jpg)
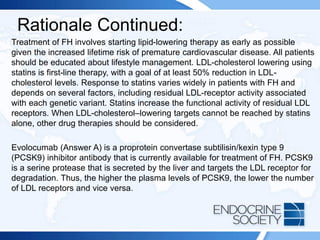
![Rationale Continued:
Use of anabolic steroids is commonly associated with low HDL-cholesterol levels.
Additionally, a paradoxical reduction in HDL cholesterol can occur with use of
fibrates (Answer B) and thiazolidinediones (eg, pioglitazone [Answer D]), either
individually or when used in combination. Such reductions are idiosyncratic and
typically occur in individuals with baseline HDL-cholesterol levels below 25 mg/dL
(<0.65 mmol/L). Polymorphisms in 1 or more genes associated with HDL
metabolism may predispose to such an effect. A sudden, dramatic decrease in HDL
cholesterol is occasionally precipitated by an underlying hematologic malignancy.
However, there is no evidence of such disorders in this patient.
Primary low HDL-cholesterol syndromes as part of monogenic disorders, although
rare in the general population, are more frequently observed in individuals with very
low HDL-cholesterol levels. Such genetic disorders occur due to mutations in the
genes encoding apolipoprotein A-I (the primary protein associated with HDL),
ABCA1 (the protein that allows cellular cholesterol to be taken up by apolipoprotein
A-I to form HDL particles), or LCAT (the enzyme that esterifies the cholesterol so
that it can move into the core of HDL).](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-379-320.jpg)
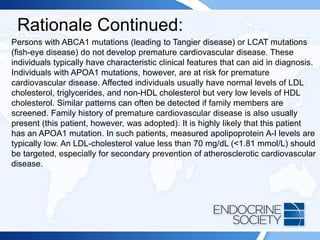


![ITE 2018 Question 73
A 51-year-old man comes to see you for care of type 2 diabetes mellitus. His
fasting lipid panel reveals the following:
• LDL cholesterol = 92 mg/dL (<100 mg/dL [optimal]) (SI: 2.38 mmol/L
[<2.59 mmol/L])
• HDL cholesterol = 42 mg/dL (>60 mg/dL [optimal]) (SI: 1.09 mmol/L
[>1.55 mmol/L])
• Triglycerides = 320 mg/dL (<150 mg/dL [optimal]) (SI: 3.62 mmol/L
[<1.70 mmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-383-320.jpg)


![Reference(s):
• Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of
diabetes on cardiovascular disease risk and all-cause mortality in older men:
influence of age at onset, diabetes duration, and established and novel risk
factors. Arch Intern Med. 2011;171(5):404-410. PMID: 21403036
• Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et
al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-
analysis of data from 170,000 participants in 26 randomised trials. Lancet.
2010;376(9753):1670-1681. PMID: 21067804
• ACCORD Study Group, Ginsberg HN, Elam MB, et al. Effects of combination
lipid therapy in type 2 diabetes mellitus [published correction appears in N
Engl J Med. 2010;362(18):1748]. N Engl J Med. 2010;362(17):1563-1574.
PMID: 20228404
• Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a
systematic review and meta-analysis. Lancet. 2010;375(9729):1875-1884.
PMID: 20462635
• Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet.
2014;384(9943):626-635. PMID: 25131982](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-387-320.jpg)





![ITE 2018 Question 14
A 32-year-old man is referred for management of chemotherapy-related
primary hypogonadism. He initially presented to his primary care physician 6
months ago with fatigue and decreased libido. After appropriate workup,
intramuscular testosterone enanthate, 200 mg every 2 weeks, was initiated.
Although he is better overall, he is bothered by fluctuation in his mood that
occurs a few days before his next injection. He also experiences fatigue at the
same time. On physical examination, his vital signs are normal. Testicular
volume is 14 mL bilaterally.
Laboratory test results:
• Testosterone (1 week after testosterone injection) = 767 ng/dL (300-900
ng/dL) (SI: 26.6 nmol/L [10.4-31.2 nmol/L])
• Hematocrit = 42.2% (41%-50%) (SI: 0.422 [0.41-0.51])
• Prostate-specific antigen = 1.0 ng/mL (<2.0 ng/mL) (SI: 1.0 µg/L [<2.0
µg/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-398-320.jpg)




![ITE 2018 Question 34
Laboratory test results:
• Total testosterone (morning) = 151 ng/dL (300-900 ng/dL) (SI: 5.2 nmol/L
10.4-31.2 nmol/L])
• LH = 2.9 mIU/mL (1.0-9.0 mIU/mL) (SI: 2.9 IU/L [1.0-9.0 IU/L])
• FSH = 3.3 mIU/mL (1.0-13.0 mIU/mL) (SI: 3.3 IU/L [1.0-13.0 IU/L])
• Prolactin = 13 ng/mL (4-23 ng/mL) (SI: 0.6 nmol/L [0.17-1.00 nmol/L])
• Transferrin saturation = 32% (14%-50%)
Pituitary MRI shows a 3-mm microadenoma without evidence of stalk
deviation.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-404-320.jpg)




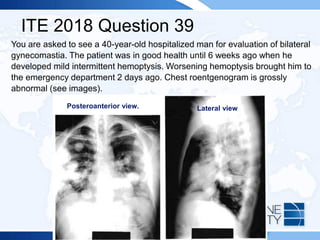


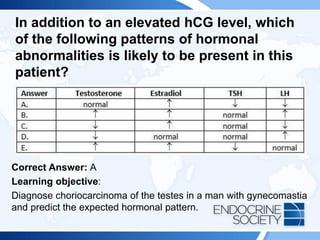

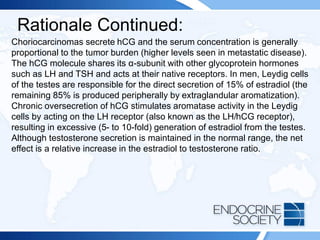


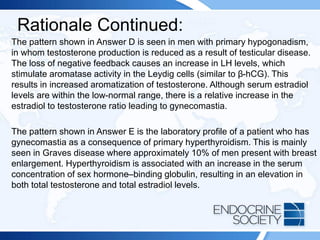



![ITE 2018 Question 55
Laboratory test results:
• Total testosterone = 150 ng/dL (300-900 ng/dL) (SI: 5.2 nmol/L [10.4-31.2
nmol/L])
• Serum prolactin = 20 ng/mL (5-20 ng/mL) (SI: 0.9 nmol/L [0.2-0.9 nmol/L])
• FSH = 3.0 mIU/mL (1.0-13.0 mIU/mL) (SI: 3.0 IU/L [1.0-13.0 IU/L])
• LH = 3.0 mIU/mL (1.0-9.0 mIU/mL) (SI: 3.0 IU/L [1.0-9.0 IU/L])
X-ray of the hands reveals chondrocalcinosis of the small joints bilaterally.
Sellar MRI reveals no pituitary mass.
Pituitary MRI shows a 3-mm microadenoma without evidence of stalk
deviation.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-421-320.jpg)





![ITE 2018 Question 66
A 42-year-old man is referred for evaluation of a low serum testosterone level.
He has been troubled by decreasing libido and low energy. He has two
teenaged children.
On physical examination, his BMI is 23 kg/m2. He has normal secondary
sexual characteristics with no gynecomastia, striae, or acne. His testicular
volume is 15 mL bilaterally.
Laboratory test results:
• Total testosterone = 150 ng/dL (300-900 ng/dL) (SI: 5.2 nmol/L [10.4-31.2
nmol/L])
• LH = 3.0 mIU/mL (1.0-9.0 mIU/mL) (SI: 3.0 IU/L [1.0-9.0 IU/L])
• FSH = 3.0 mIU/mL (1.0-13.0 mIU/mL) (SI: 3.0 IU/L [1.0-13.0 IU/L])
Sellar CT is normal.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-427-320.jpg)








![ITE 2018 Question 4
Laboratory test results:
• Sodium = 137 mEq/L (136-142 mEq/L) (SI: 137 mmol/L [136-142 mmol/L])
• Potassium = 4.4 mEq/L (3.5-5.0 mEq/L) (SI: 4.4 mmol/L [3.5-5.0 mmol/L])
• Creatinine = 0.7 mg/dL (0.6-1.1 mg/dL) (SI: 61.9 µmol/L [53.0-97.2
µmol/L])
• TSH = 2.1 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 1.4 ng/dL (0.8-1.8 ng/dL) (SI: 18.0 pmol/L [10.3-23.17 pmol/L])
• GH = <0.01 ng/mL (0.01-3.61 ng/mL) (SI: <0.01 mg/L [0.01-3.61 mg/L])
• IGF-1 = <50 ng/mL (106-277 ng/mL) (SI: 6.6 nmol/L [13.9-36.3 nmol/L])
• DHEA-S = 14 µg/dL (31-228 µg/dL) (SI: 0.38 µmol/L [0.84-6.78 µmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-438-320.jpg)









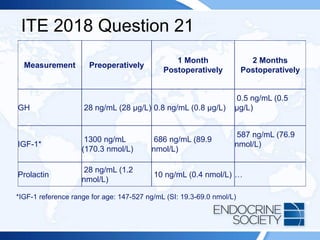






![ITE 2018 Question 31
Laboratory test results:
• Sodium = 137 mEq/L (136-142 mEq/L) (SI: 137 mmol/L [136-142
mmol/L])
• Potassium = 4.0 mEq/L (3.5-5.0 mEq/L) (SI: 4.0 mmol/L [3.5-5.0
mmol/L])
• Serum urea nitrogen = 21 mg/dL (8-23 mg/dL) (SI: 7.5 mmol/L [2.9-8.2
mmol/L])
• Creatinine = 1.1 mg/dL (0.7-1.3 mg/dL) (SI: 97.2 µmol/L [61.9-114.9
µmol/L])
The intensive care unit team has just performed a 250-mcg ACTH simulation
test. The baseline cortisol value was 3.9 µg/dL (107.6 nmol/L). Sixty minutes
after administration of 250 mcg of ACTH, it was 24.1 µg/dL (664.9 nmol/L).](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-457-320.jpg)



![ITE 2018 Question 35
A 31-year-old woman first presented 4 years earlier with amenorrhea and
galactorrhea. An 11-mm prolactinoma was identified, and she has since
been treated with cabergoline. Her current dosage is 0.5 mg twice weekly.
She has regular menses and no galactorrhea. She has recently married and
wishes to become pregnant as soon as possible; she is using no
contraception.
On physical examination, she appears well. Her height is 64 in (162.6 cm),
and weight is 140 lb (63.6 kg) (BMI = 24 kg/m2). Her blood pressure is
105/68 mm Hg. No abnormalities are noted.
Laboratory test results:
• Prolactin = 26 ng/mL (4-30 ng/mL) (SI: 1.13 nmol/L [0.17-1.30 nmol/L])
• β-hCG = 2.1 mIU/mL (<3.0 mIU/mL) (SI: 2.1 mIU/mL [<3.0 IU/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-463-320.jpg)
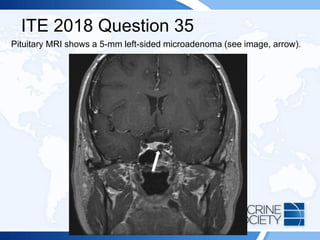




![ITE 2018 Question 46
A 43-year-old woman is referred for management of Cushing disease
manifested by typical signs and symptoms.
Laboratory test results:
• Urinary free cortisol = 451 µg/24 h (4-50 µg/24 h) (SI: 1244.8 nmol/d
[11.0-138.0 nmol/d])
• Serum cortisol (8 AM) = 28 µg/dL (5-25 µg/dL) (SI: 772.5 nmol/L [137.9-
689.7 nmol/L])
• ACTH = 93 pg/mL (10-60 pg/mL) (SI: 20.5 pmol/L [2.2-13.2 pmol/L])
Following incomplete surgical removal of her 9-mm pituitary adenoma, her
hormone levels remain elevated. While on medical therapy, the patient
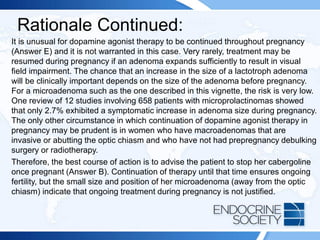
subsequently develops diabetes mellitus.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-470-320.jpg)

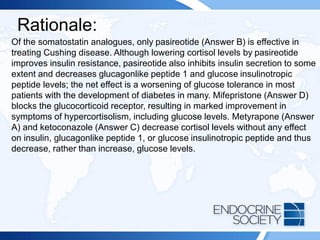
![Reference(s):
• Colao A, Petersenn S, Newell-Price J, et al; Pasireotide B2305 Study
Group. A 12-month phase 3 study of pasireotide in Cushing disease
[published correction appears in N Engl J Med. 2012;367(8):780]. N
Engl J Med. 2012;366(10):914-924. PMID: 22397653
• Wallia A, Colleran K, Prunell JQ, Gross C, Molitch ME. Improvement in
insulin sensitivity during mifepristone treatment of Cushing syndrome:
early and late effects. Diabetes Care. 2013;36(9):E147-E148. PMID:
23970725
• Molitch ME. Current approaches to the pharmacological management
of Cushing's disease. Mol Cell Endocrinol. 2015;408:185-189. PMID:
2540859](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-473-320.jpg)
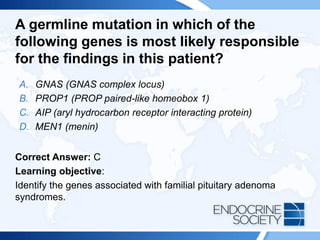
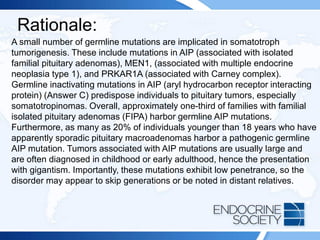
![ITE 2018 Question 48
A 19-year-old man is referred for gigantism. His height is 82 in (208.3 cm),
and his weight is 273 lb (124.1 kg) (BMI = 28.5 kg/m2), His hands and feet
are enlarged, and he has prognathism. A maternal uncle was thought to
have had a pituitary adenoma of uncertain type. There is no known family
history of calcium disorders or kidney stones.
Laboratory test results:
• Random GH = 90 ng/mL (0.01-0.97 ng/mL) (SI: 90 µg/L [0.01-0.97 µg/L])
• Serum IGF-1 = 1233 ng/mL (147-527 ng/mL) (SI: 161.5 nmol/L [19.3-
69.0 nmol/L])
• Serum calcium, normal
MRI shows a 4.3-cm pituitary adenoma with suprasellar extension.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-474-320.jpg)

![ITE 2018 Question 60
Following transsphenoidal surgery, a 56-year-old woman with acromegaly
has residual tumor in the clivus.
Postoperative laboratory test results:
• GH = 9.0 ng/mL (0.01-3.61 ng/mL) (SI: 9.0 µg/L [0.01-3.61 µg/L])
• IGF-1 = 490 ng/mL (78-220 ng/mL) (SI: 64.2 nmol/L [10.2-28.8 nmol/L])
Her GH and IGF-1 levels decrease by about 10% with administration of
octreotide LAR, 30 mg every 4 weeks, and she remains symptomatic.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-479-320.jpg)



![ITE 2018 Question 64
During her third month of pregnancy, a 26-year-old woman has developed
hypertension; diabetes mellitus; hirsutism; and wide, purple striae on her
abdomen.
Laboratory test results:
• Serum cortisol (8 AM) = 37 μg/dL (5-25 μg/dL [nonpregnant patients])
(SI: 1020.8 nmol/L [137.9-689.7 nmol/L])
• ACTH = 129 pg/mL (10-60 pg/mL) (SI: 28.4 pmol/L [2.2-13.2 pmol/L])
• Urinary free cortisol = 475 µg/24 h (4-50 µg/24 h [nonpregnant patients])
(SI: 1311 nmol/d [11-138 nmol/d])
MRI shows a 6-mm pituitary adenoma.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-483-320.jpg)








![ITE 2018 Question 3
She has no specific concerns except for difficulty sleeping characterized by early
awakening and inability to get back to sleep. She has osteoporosis treated with a
bisphosphonate, but no other medical problems. Her levothyroxine dosage is 137
mcg daily (2.3 mcg/kg as a weight-based dosage).
On physical examination, there is no palpable tissue in the thyroid bed and no
cervical adenopathy.
Laboratory test results:
• TSH = 0.09 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 1.8 ng/dL (0.8-1.8 ng/dL) (SI: 23.2 pmol/L [10.30-23.17 pmol/L])
• Serum thyroglobulin = <0.1 ng/mL (<1.0 ng/mL) (SI: <0.1 µg/L [<0.1 µg/L])
• Antithyroglobulin antibodies, negative
Cervical ultrasonography shows absence of tissue in the thyroid bed and some
subcentimeter, benign-appearing lymph nodes bilaterally.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-493-320.jpg)



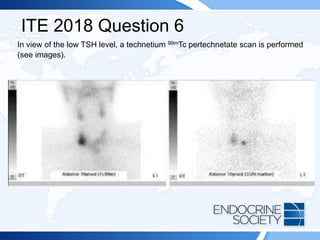
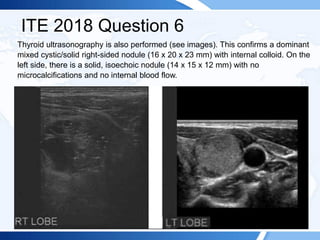
![ITE 2018 Question 6
A 72-year-old man is referred to you by his primary care physician because of
abnormal thyroid function test results and a right-sided neck mass. The patient
reports no symptoms of thyroid dysfunction and was not aware of the neck mass
until his physician noted it.
On physical examination, his blood pressure is 134/76 mm Hg and pulse rate is 76
beats/min. Examination of his neck confirms a right-sided neck mass (2 cm in
maximal diameter) that moves with swallowing. There is no palpable cervical
lymphadenopathy. There is no tremor and no obvious signs of thyroid-related
ophthalmopathy.
Laboratory test results:
• TSH = 0.06 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 1.6 ng/dL (0.8-1.8 ng/dL) (SI: 20.6 pmol/L [10.30-23.17 pmol/L])
• Free T3 = 4.0 pg/mL (2.3-4.2 pg/mL) (SI: 6.1 pmol/L [3.53-6.45 pmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-499-320.jpg)









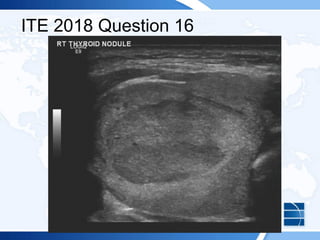



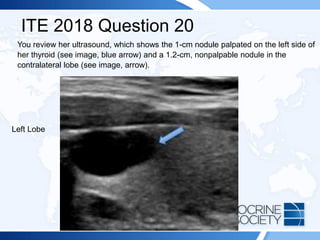
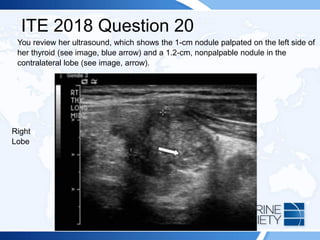
![ITE 2018 Question 20
A 25-year-old woman presents for further evaluation and management of a left-sided
thyroid nodule that was palpated by her gynecologist during a visit for an annual
examination. The gynecologist had ordered thyroid function testing and thyroid
ultrasonography and referred her to you. The patient has no history of thyroid
dysfunction, no symptoms suggestive of either hypothyroidism or hyperthyroidism,
and no compressive symptoms.
On visual inspection, you note that her thyroid gland is asymmetric, with the left lobe
being larger than the right. You palpate a mobile, soft, 1-cm nodule within the left
lobe of the thyroid. Otherwise, she has no abnormal examination findings.
Thyroid function test results:
• TSH = 1.2 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 1.3 ng/dL (0.8-1.8 ng/dL) (SI: 16.7 pmol/L [10.30-23.17 pmol/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-521-320.jpg)




![ITE 2018 Question 26
A 39-year-old woman presents with a new 10-lb (4.5-kg) weight gain, constipation,
irregular menses, and cold intolerance. She gets up very early in the morning for her job
as a school bus driver and reports that she is exhausted by early afternoon. She has
been feeling so poorly that she has called in sick to work for the past 4 days.
Laboratory test results (sample collected while fasting):
• TSH = 167 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 0.2 ng/dL (0.8-1.8 ng/dL) (SI: 2.6 pmol/L [10.3-23.2 pmol/L])
• TPO antibodies = 692 IU/mL (<2.0 IU/mL) (SI: 692 kIU/L [<2.0 kIU/L])
• Total cholesterol = 292 mg/dL (<200 mg/dL [optimal]) (SI: 7.56 mmol/L [<5.18
mmol/L])
• LDL cholesterol = 201 mg/dL (<100 mg/dL [optimal]) (SI: 5.21 mmol/L [<2.59 mmol/L])
• Triglycerides = 195 mg/dL (<150 mg/dL [optimal]) (SI: 2.20 mmol/L [<3.88 mmol/L])
• HDL cholesterol = 52 mg/dL (>60 mg/dL [optimal]) (SI: 1.35 mmol/L [>1.55 mmol/L])
Levothyroxine is started.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-529-320.jpg)



![ITE 2018 Question 29
A 24-year-old man with type 1 diabetes mellitus presents with fatigue and
abdominal discomfort.
Laboratory test results:
• Serum TSH = 15.2 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 0.7 ng/dL (0.8-1.8 ng/dL) (SI: 9.0 pmol/L [10.3-23.2 pmol/L])
• TPO antibodies, positive
Replacement therapy with levothyroxine is initiated, but 2 weeks later he
reports feeling worse than ever. On physical examination, the patient’s blood
pressure is 106/74 mm Hg and pulse rate is 104 beats/min.
Repeated thyroid function testing:
• Serum TSH = 5.8 mIU/L
• Free T4 = 1.2 ng/dL (SI: 15.4 pmol/L)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-533-320.jpg)



![ITE 2018 Question 30
A 37-year-old woman presents for ongoing management of hyperthyroidism. Graves
disease was diagnosed 3 months ago when she presented with typical symptoms
and signs of thyrotoxicosis. She did not report any symptoms of ophthalmopathy and
there was no clinical evidence of thyroid eye disease at presentation.
Laboratory test results at diagnosis:
• Serum TSH = <0.01 mIU/L (0.5-5.0 mIU/L)
• Serum free T4 = 3.5 ng/dL (0.8-1.8 ng/dL) (SI: 45.0 pmol/L [10.30-23.17 pmol/L])
• Serum free T3 = 9.8 pg/mL (2.3-4.2 pg/mL) (SI: 15.1 pmol/L [3.53-6.45 pmol/L])
• TSH receptor antibodies, markedly elevated
She smokes 10 cigarettes daily (she started smoking at age 18 years).](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-537-320.jpg)






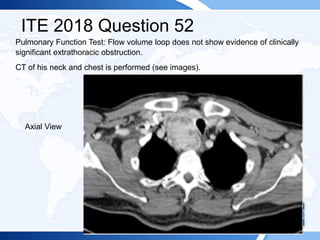
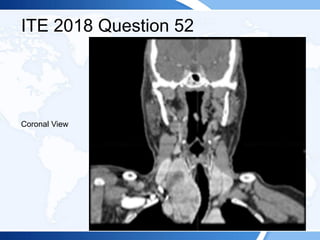
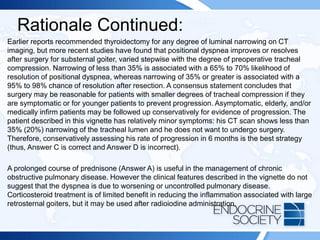
![ITE 2018 Question 52
A 72-year-old man is referred after the detection of a retrosternal goiter with tracheal
deviation on a chest radiograph. The patient has a 6-month history of mild positional
dyspnea at rest and intermittent orthopnea. He reports no symptoms of stridor,
dysphagia, or dysphonia. He has a longstanding history of mild chronic obstructive
pulmonary disease and is a former cigarette smoker.
On physical examination, he has a multinodular goiter in the neck that is palpable in
a neutral position and visible in a supine position with neck extension. The lower lobe
of the thyroid gland extends below the sternal notch and cannot be palpated. The
Pemberton sign is negative. There is tracheal deviation to the left. The rest of his
examination findings are normal.
Laboratory test results:
• Serum TSH = 3.5 mIU/L (0.5-5.0 mIU/L)
• Serum free T4 = 1.2 ng/dL (0.8-1.8 ng/dL) (SI: 15.4 pmol/L [10.30-23.17 pmol/L])
• TPO antibodies = 18 IU/mL (<2.0 IU/mL) (SI: 18 kIU/L [<2.0 kIU/L]](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-545-320.jpg)






![ITE 2018 Question 54
A 78-year-old man is seen by his primary care physician for his annual check-up.
Generally, he feels well and has no specific concerns, except for a gradual decrease
in his energy level and strength. His only chronic medical problems are hypertension
and osteoarthritis.
On physical examination, his weight is unchanged from 1 year ago and his BMI is
normal. His blood pressure is 134/80 mm Hg. Palpation of the neck reveals a
normal-sized, slightly firm thyroid gland. His examination findings are otherwise
unremarkable aside from osteoarthritis in his hands.
Laboratory test results:
• Serum sodium = 134 mEq/L (136-142 mEq/L) (SI: 134 mmol/L [136-142 mmol/L])
• Other serum electrolytes, normal
• Total cholesterol = 180 mg/dL (<200 mg/dL [optimal]) (SI: 4.66 mmol/L [<5.18 mmol/L])
• LDL cholesterol = 129 mg/dL (<100 mg/dL [optimal]) (SI: 3.34 mmol/L [<2.59 mmol/L])
• TSH = 5.7 mIU/L (0.5-5.0 mIU/L)](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-555-320.jpg)
![ITE 2018 Question 54
Laboratory test results 2 months later:
• TSH = 5.9 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 1.0 ng/dL (0.8-1.8 ng/dL) (SI: 12.9 pmol/L [10.30-23.17 pmol/L])
• TPO antibodies = <2.0 IU/mL (<2.0 IU/mL) (SI: <2.0 kIU/L [<2.0 kIU/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-556-320.jpg)

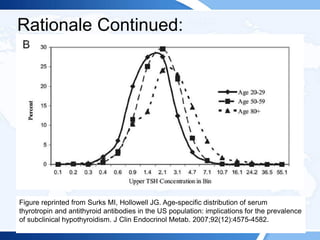


![ITE 2018 Question 57
A 58-year-old woman with stage IV medullary thyroid cancer is referred for
consideration of further therapy. Medullary thyroid cancer was diagnosed 8
years earlier and she had a persistent postoperative serum calcitonin
elevation. Distant metastases to the lungs and ribs were detected 1 year
ago, with disease progression over the past 6 months. Physical examination
reveals a well-healed thyroidectomy scar, but findings are otherwise
unremarkable.
Laboratory test results:
• Serum calcitonin = 15,000 pg/mL (<8 pg/mL) (SI: 4380 pmol/L [<2.34
pmol/L])
• Carcinoembryonic antigen = 65 ng/mL (<2.5 ng/mL) (SI: 65 µg/L [<2.5
µg/L])](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-563-320.jpg)

![Rationale Continued:
Radiolabeled anti-CEA antibodies (not calcitonin antibodies [Answer A]) have been
evaluated in medullary thyroid cancer and certain gastrointestinal tumors, with less
than impressive results. Cytotoxic chemotherapy (Answer B) has been applied to
therapy for metastatic medullary thyroid cancer, with limited utility; most regimens
involve dacarbazine combined with a second agent (not adriamycin and cisplatin).
Focal radiotherapy (Answer C) will not delay the systemic progression of metastatic
disease in this patient. There is no role for whole-brain irradiation (Answer E) in this
case.](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-566-320.jpg)
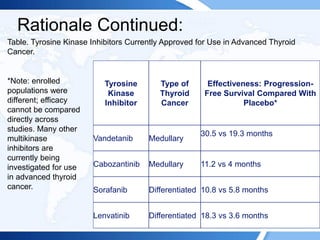

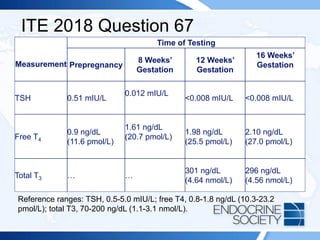
![ITE 2018 Question 67
Serum thyroglobulin is in the mid-normal range. At 12 weeks’ gestation, the
quantitative β-hCG level was 190,450 mIU/mL (190,450 IU/L) (reference
range for 12 weeks’ gestation, 27,832-210,612 mIU/mL [27,832-210,612
IU/L]).](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-571-320.jpg)

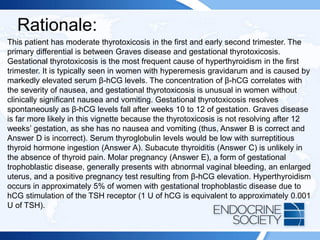

![ITE 2018 Question 87
A 66-year-old man is taking amiodarone for refractory atrial fibrillation.
Baseline thyroid function tests are normal, but one year after starting
amiodarone he develops thyrotoxicosis. On physical examination, the
patient has a pulse rate of 92 beats/min, no goiter or thyroid tenderness, a
normal lung examination, and no edema.
Laboratory test results:
• Serum TSH = <0.01 mIU/L (0.5-5.0 mIU/L)
• Free T4 = 4.3 ng/mL (0.8-1.8 ng/mL) (SI: 55.3 pmol/L [10.3-23.2 pmol/L])
• Total T3 = 215 ng/dL (70-200 ng/dL) (SI: 215 nmol/L [1.1-3.1 nmol/L])
• Radioactive iodine uptake, <1%](https://image.slidesharecdn.com/esapite-2018-250122125359-eae038a1/85/ESAP-ITE-for-the-year-2018-slide-deck-slides-575-320.jpg)