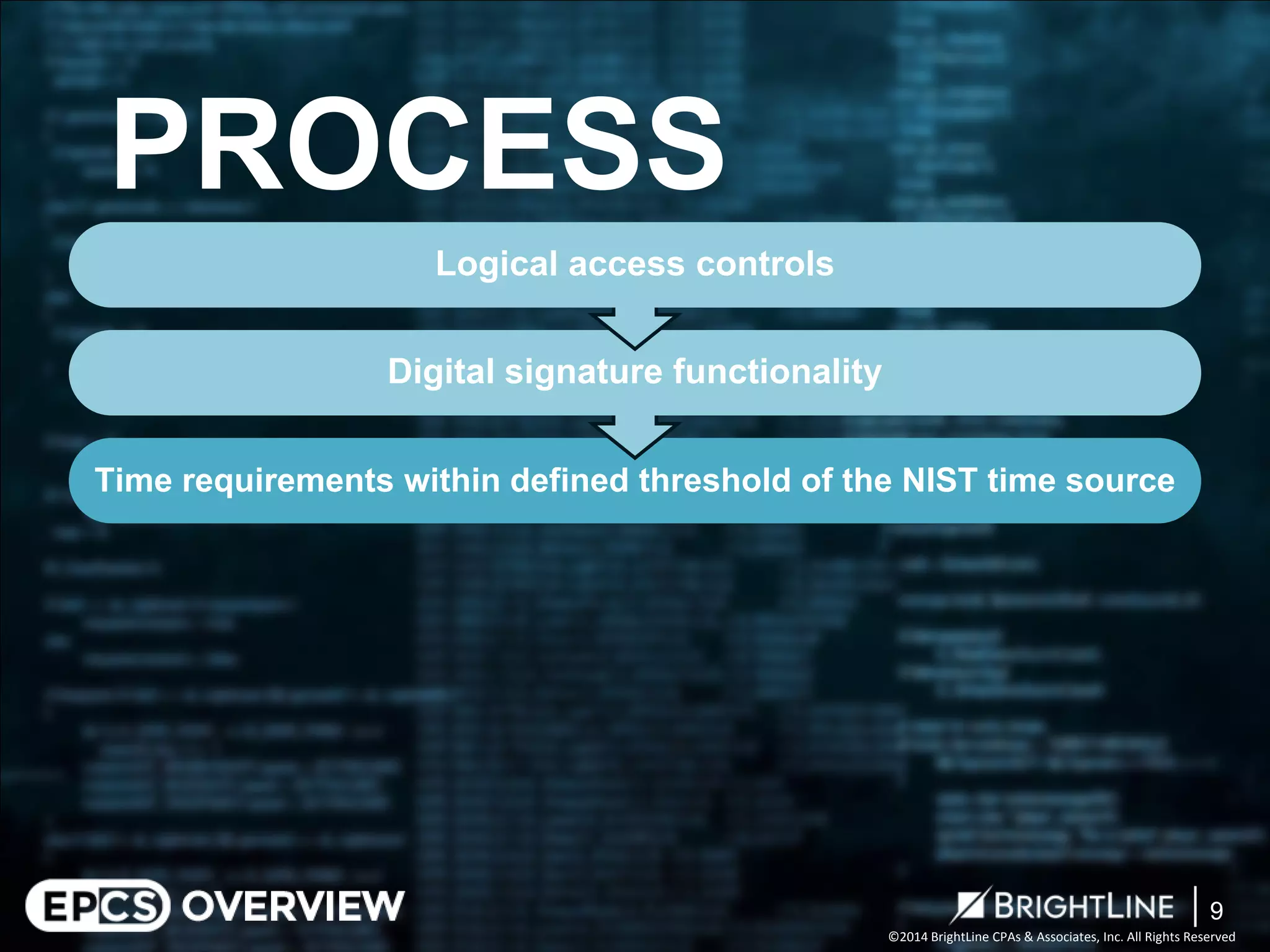
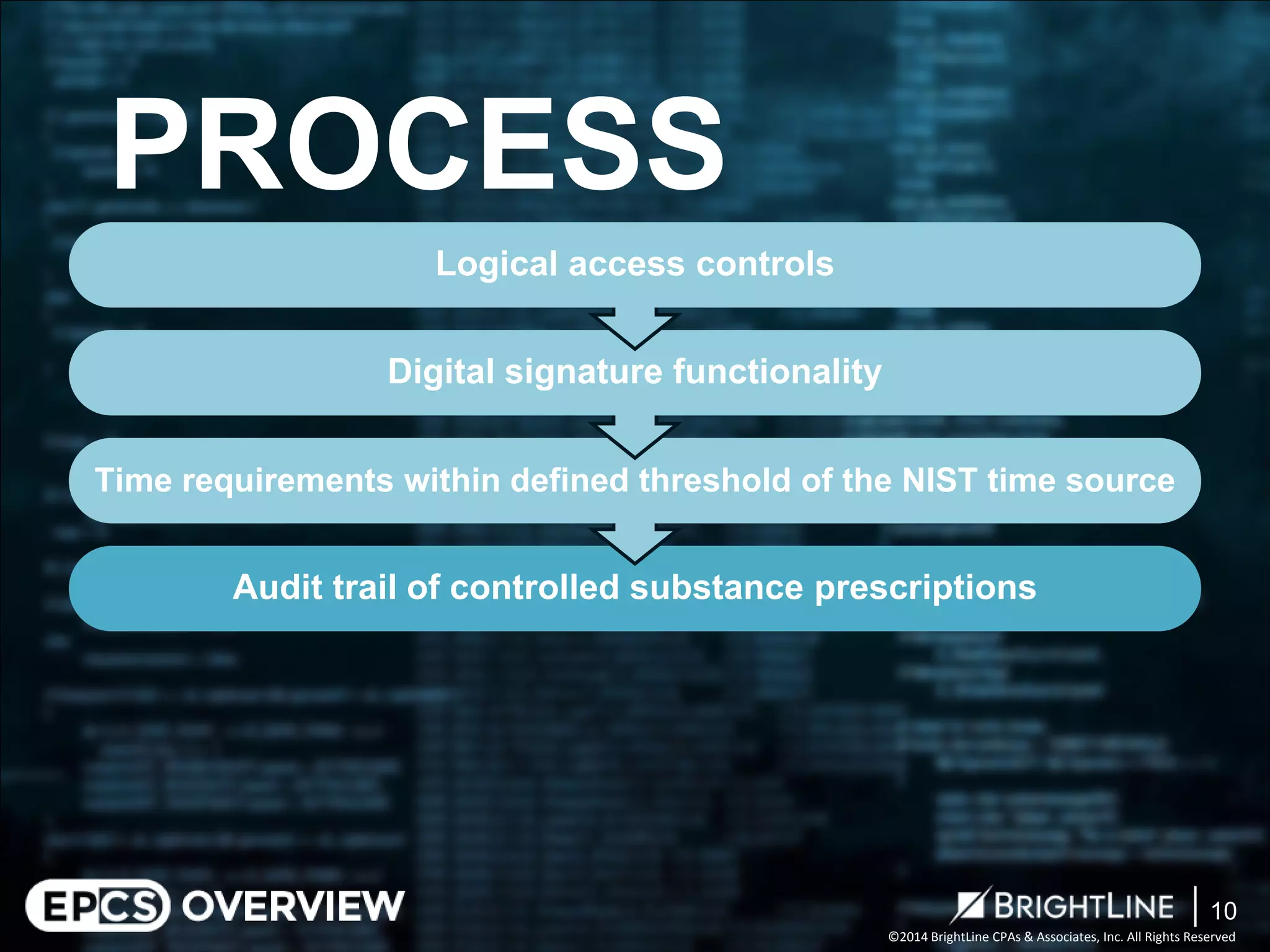
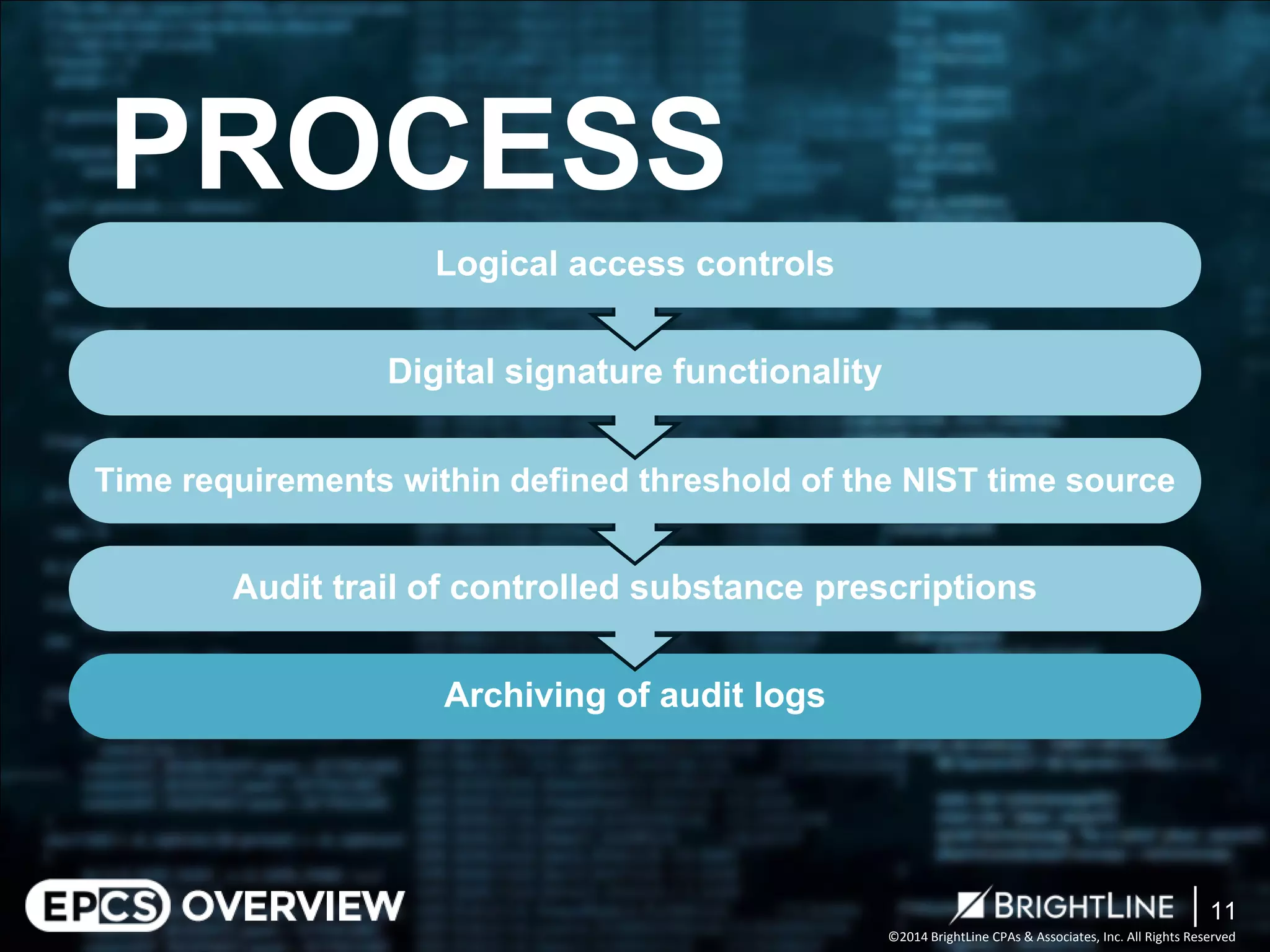
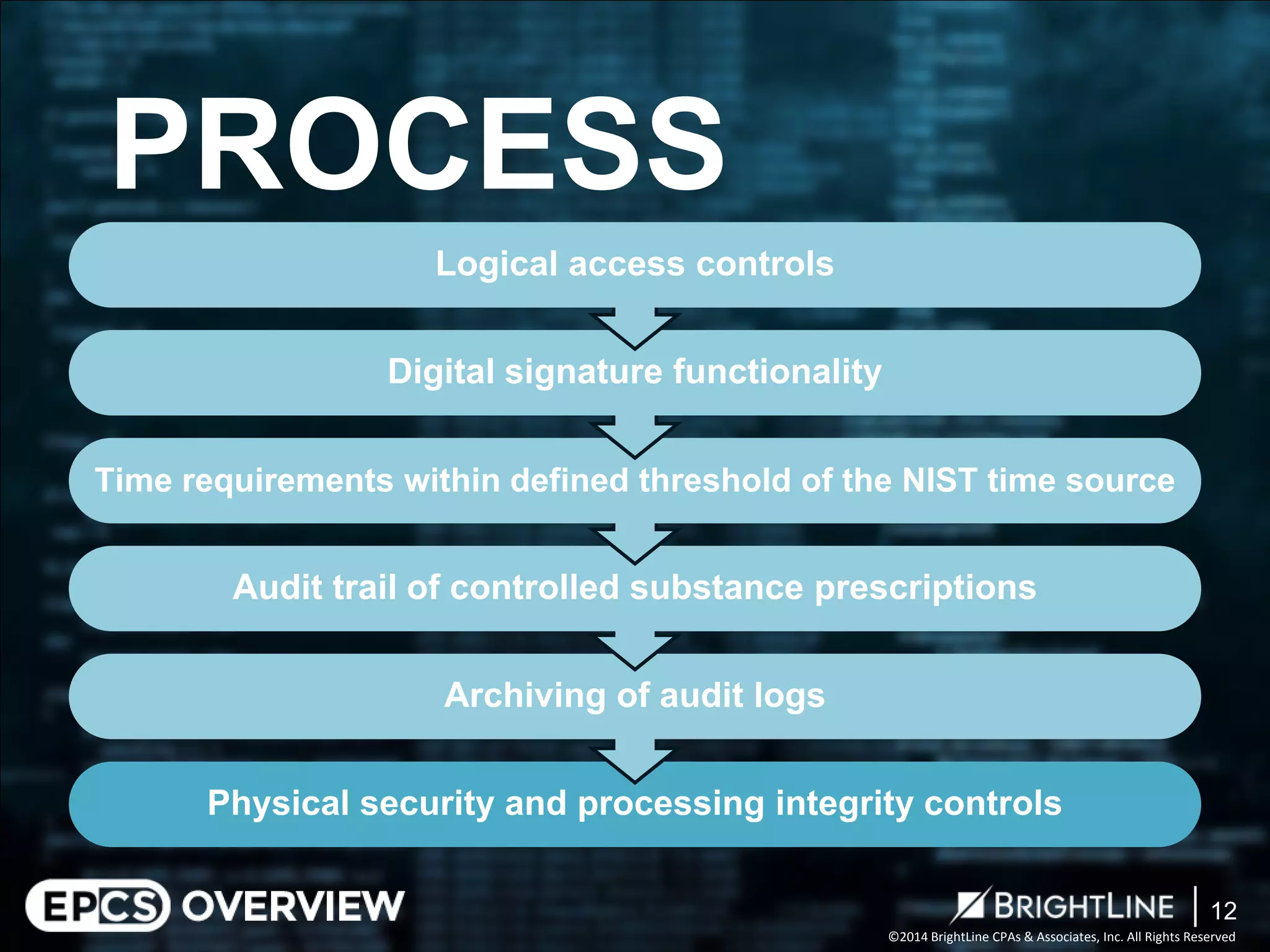
The document outlines the Electronic Prescriptions for Controlled Substances (EPCS) process following the DEA's 2010 revisions that allow prescriptions to be issued electronically, aiming to reduce paperwork and forgery risks. It clarifies compliance requirements for practitioners and pharmacies, including the need for certification and adherence to specific DEA regulations. The document also describes audit phases to ensure compliance with the DEA requirements, with a focus on evaluating controls and areas for process improvement.