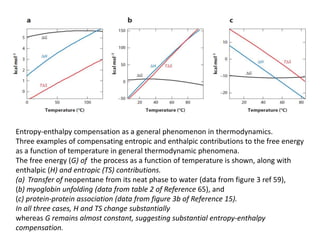
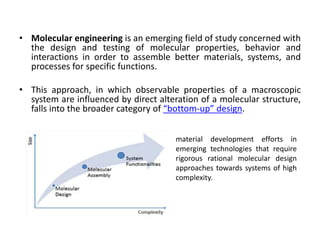
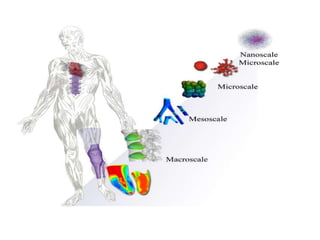
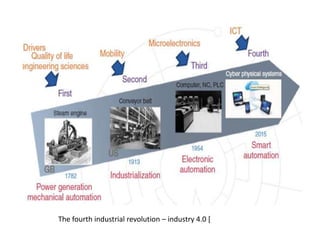
Three examples of entropy-enthalpy compensation in general thermodynamic phenomena are summarized.
1) The transfer of neopentane from its neat phase to water shows substantial entropy and enthalpy changes that compensate each other, keeping the free energy nearly constant.
2) Myoglobin unfolding and protein-protein association also demonstrate large compensating entropy and enthalpy changes with a nearly constant free energy.
3) In all three cases, the entropy and enthalpy change substantially while the free energy remains almost constant, suggesting significant entropy-enthalpy compensation.