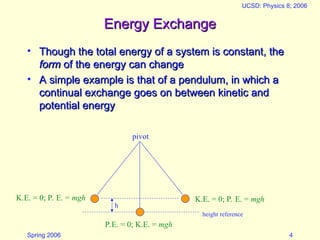
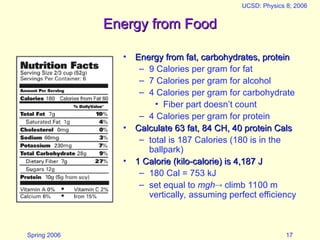
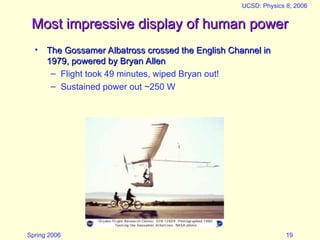
The document discusses the concept of energy in physics, focusing on its various forms, conservation, and transformation. It explains that energy cannot be created or destroyed, presenting examples of energy conversion in everyday scenarios and the relationship between energy and heat. Additionally, it addresses human energy requirements, efficiency, and the impact of factors like air resistance on energy consumption.