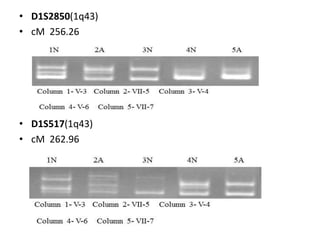
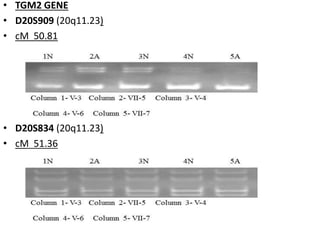
The document discusses the mapping of autosomal recessive genes related to ectodermal dysplasias and hereditary genodermatosis in Azad Jammu and Kashmir. It outlines the classification of these genetic disorders, research objectives, family pedigrees, and results from genetic testing showing specific genes involved in ectodermal dysplasias. The study aims to provide genetic counseling and establish diagnostic strategies for affected families to reduce the prevalence of these disorders in the region.