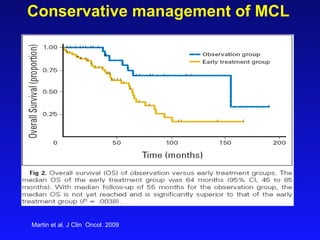
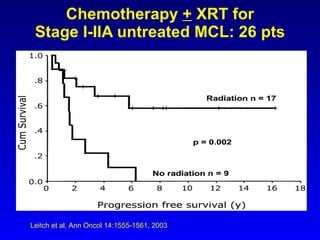
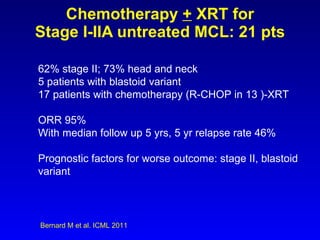
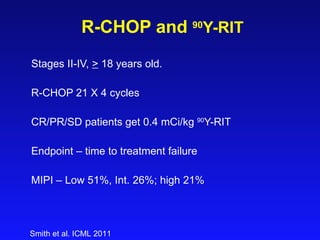
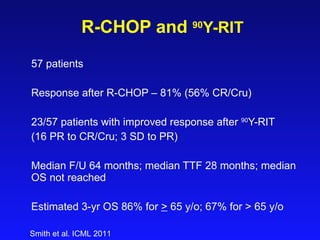
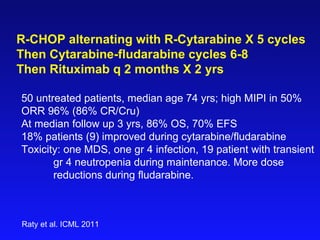
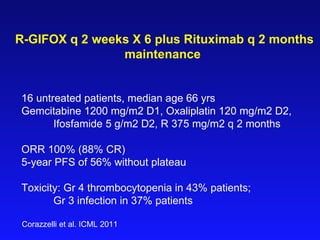
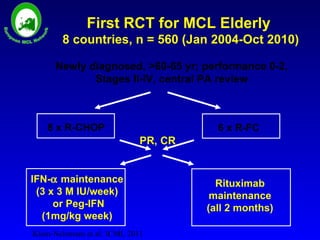
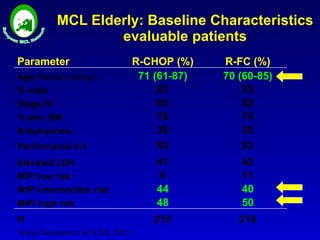
This document summarizes strategies for treating mantle cell lymphoma (MCL), including:
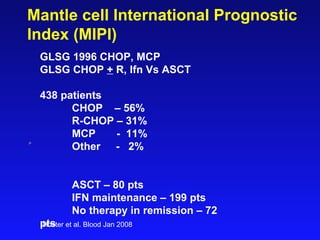
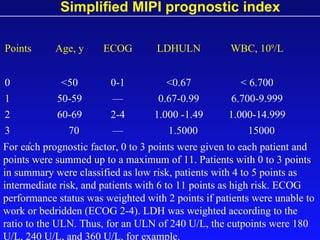
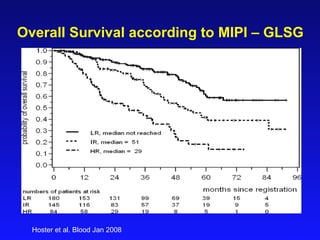
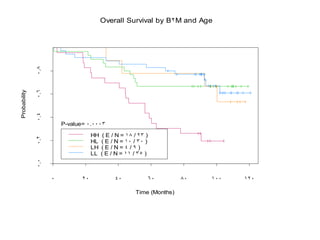
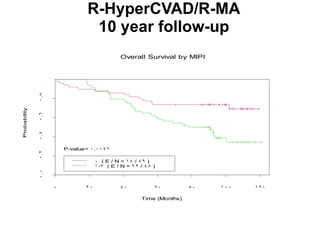
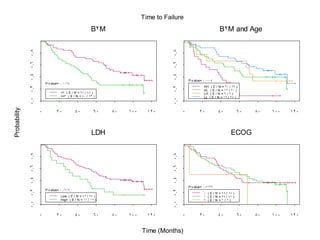
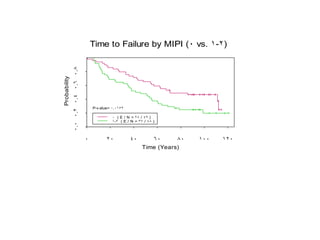
- When to treat: stratifying based on prognostic factors like the Mantle Cell International Prognostic Index (MIPI)
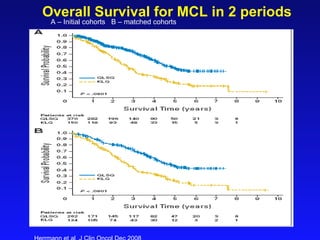
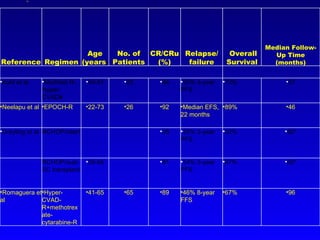
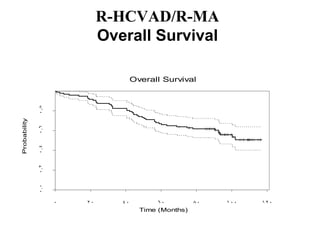
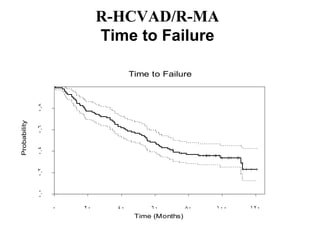
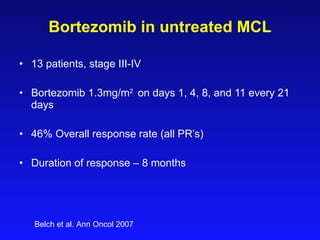
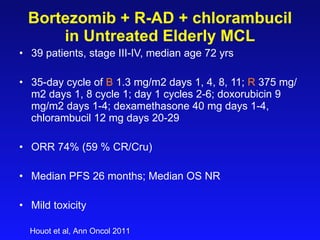
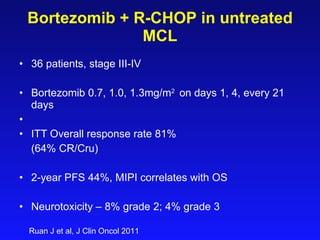
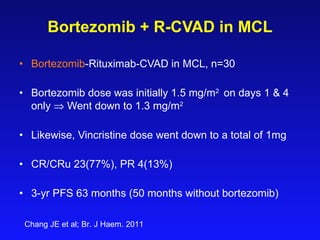
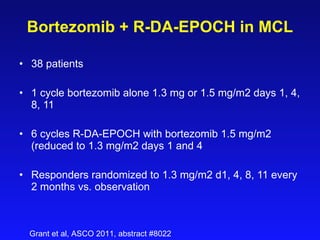
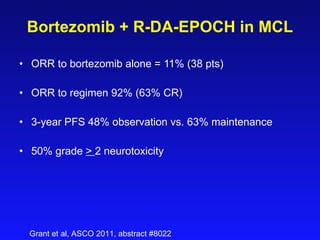
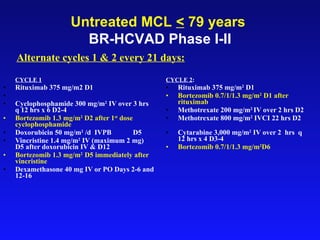
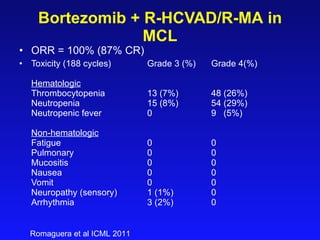
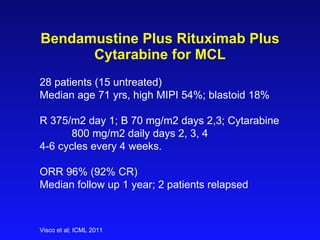
- What to treat with: conventional chemotherapy like R-CHOP plus newer drugs like bortezomib; more intensive regimens including Hyper-CVAD have shown improved survival
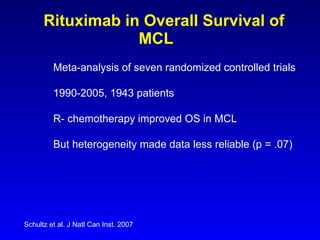
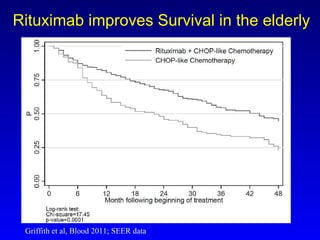
- Rituximab improves outcomes and its addition to chemotherapy is recommended
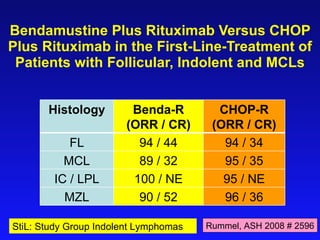
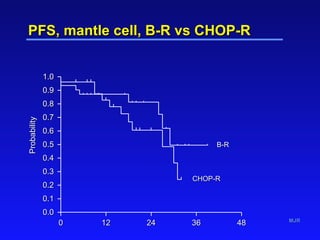
- Bendamustine plus rituximab has efficacy comparable to R-CHOP for MCL