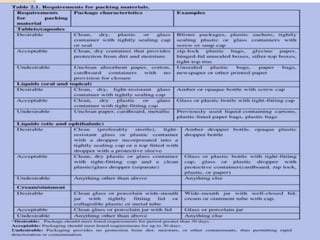
The document provides detailed guidelines for the dispensing of medications, specifically cloxacillin, including usage instructions, precautions, labeling, counting, packaging, and counseling points for both patients and pharmacy professionals. It emphasizes the importance of maintaining the quality of medications and proper handling practices to minimize errors and ensure patient safety. Additionally, it outlines necessary documentation procedures for prescriptions, including record-keeping for accountability and inventory management.