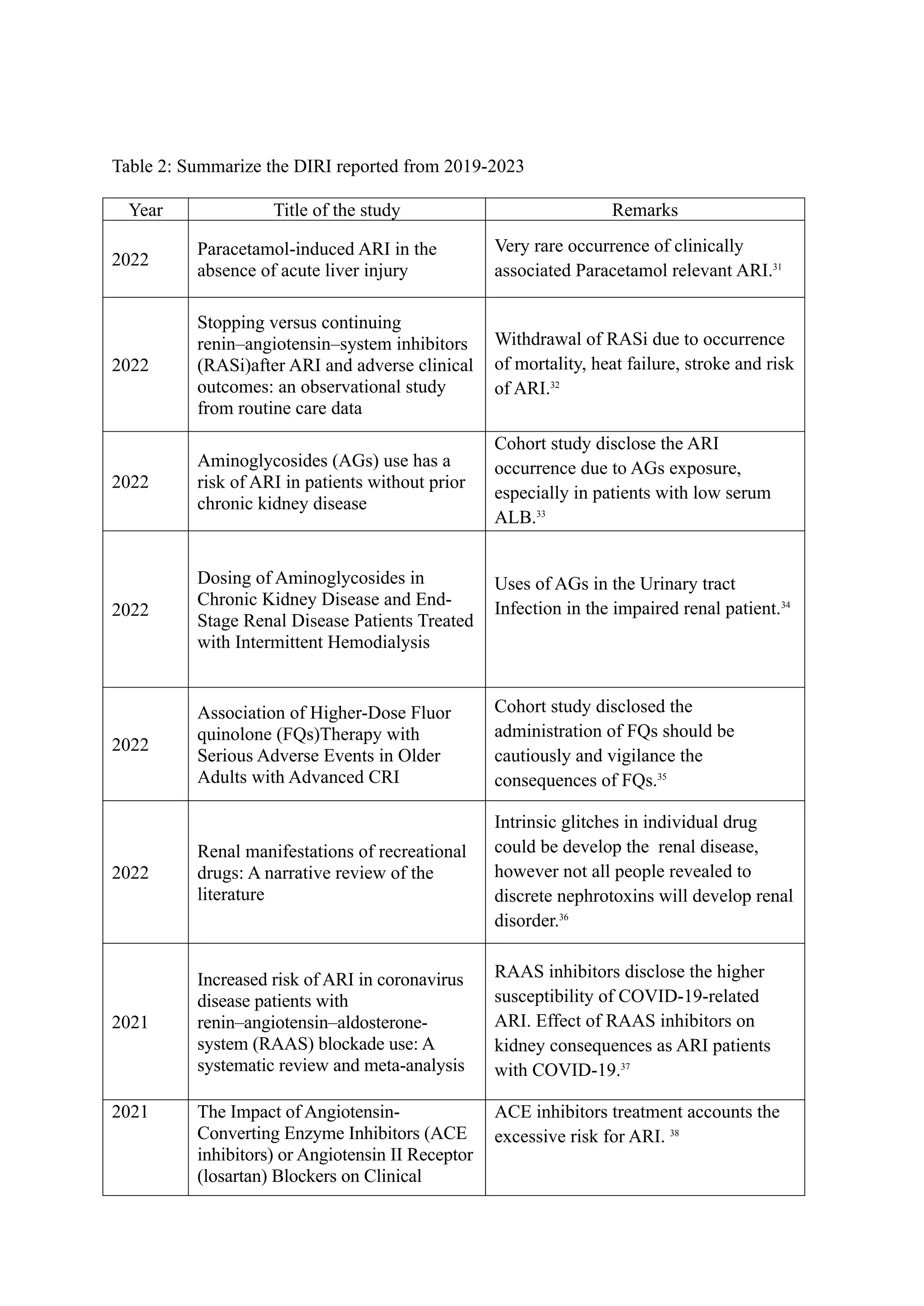
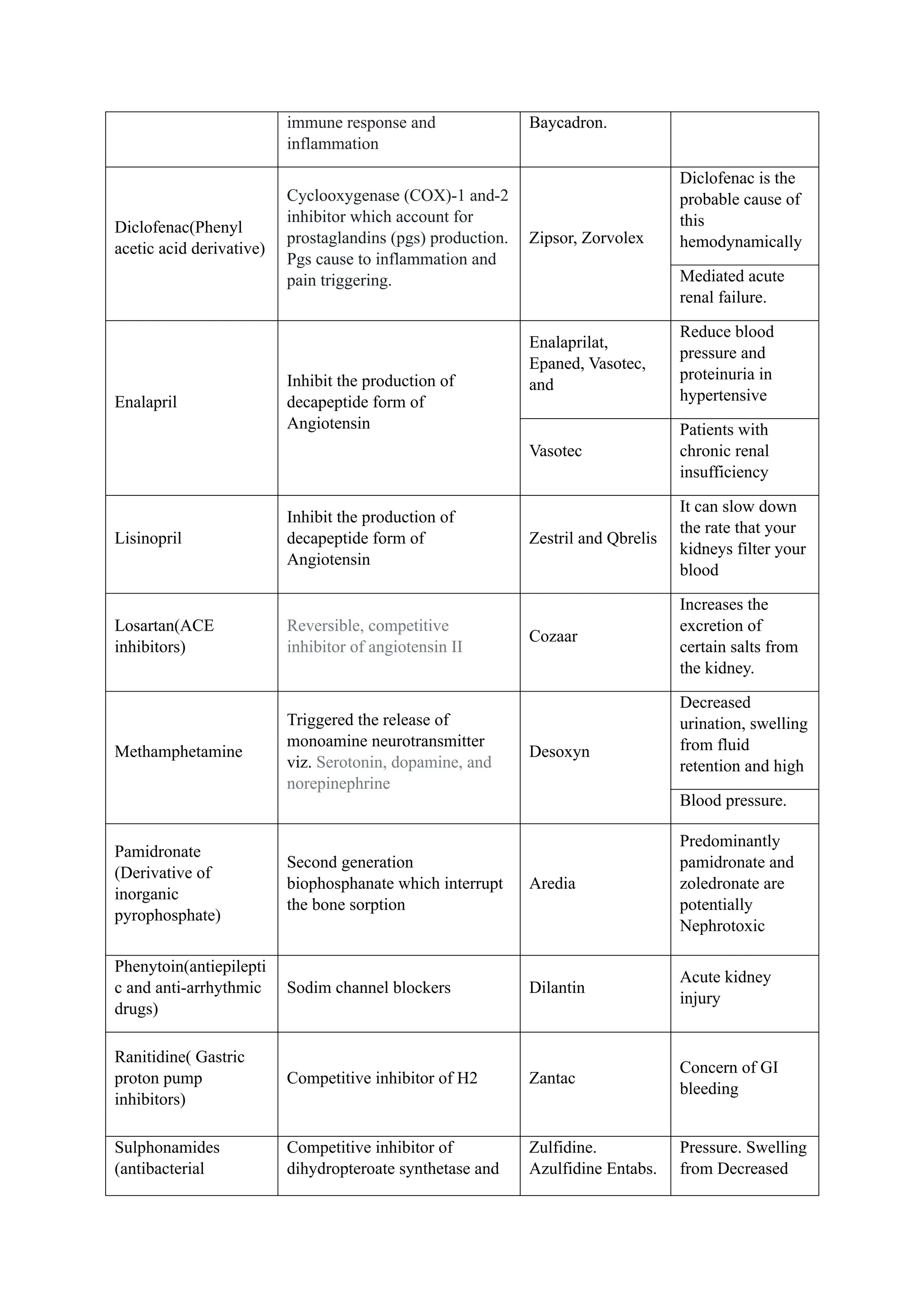
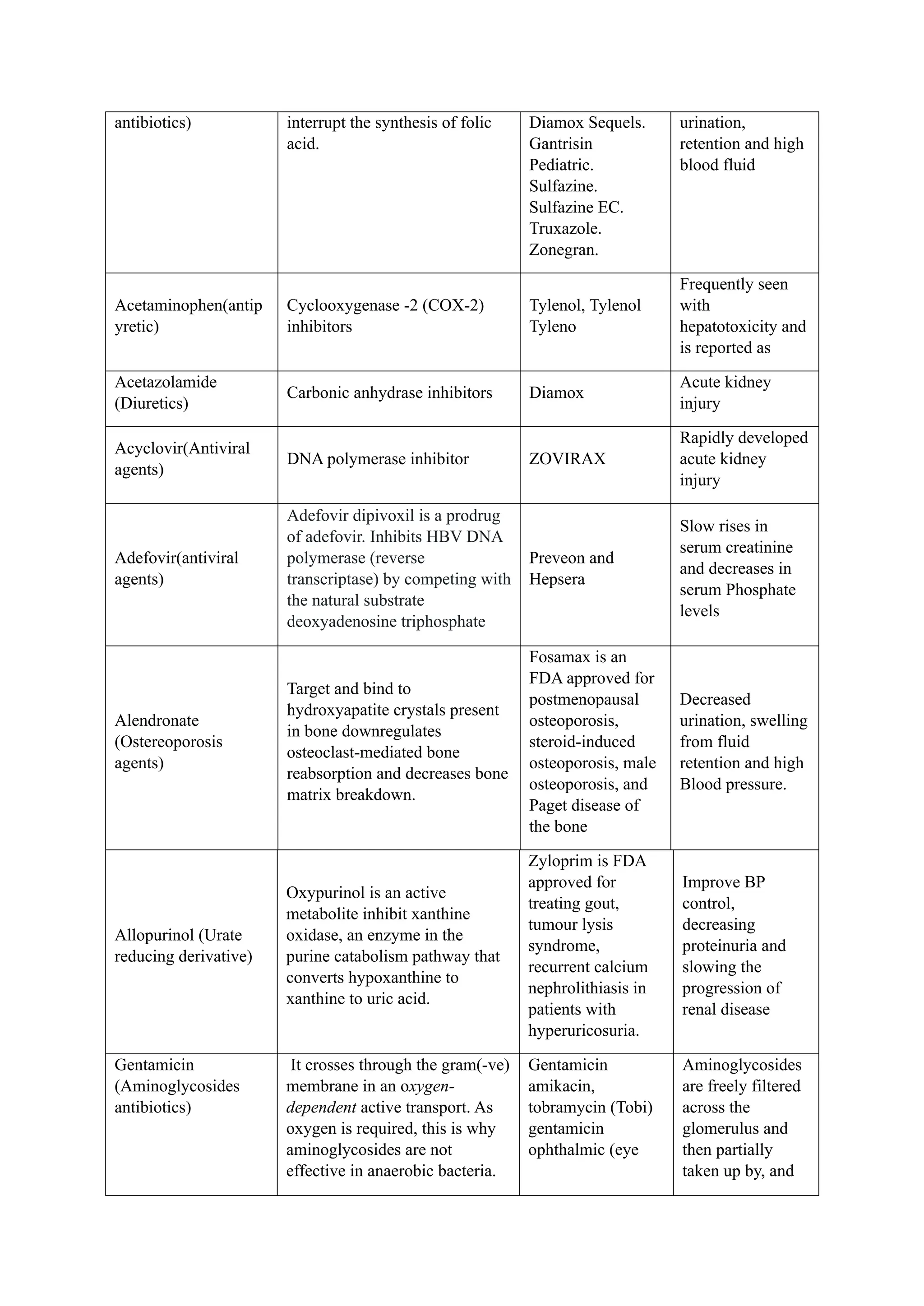
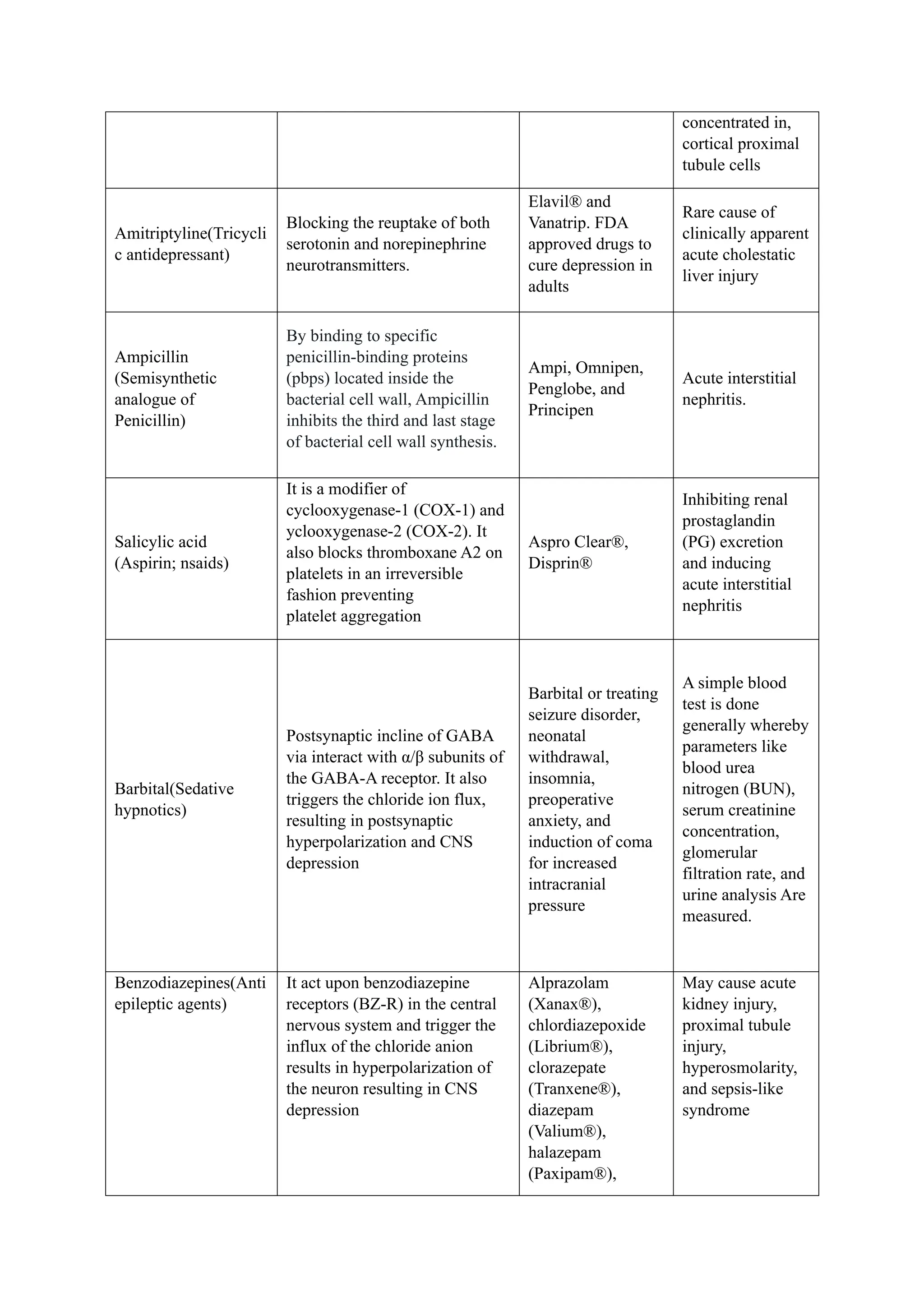
The document reviews drug-induced renal injury (DIRI), highlighting its prevalence, contributing medications, and the complexities of diagnosis and treatment. It emphasizes the risk factors, biomarkers, and clinical consequences associated with renal impairment due to various pharmacological agents, including both conventional and herbal medications. The review also delineates a novel approach termed the '6R's for managing DIRI, advocating for early detection and intervention strategies to mitigate kidney damage.










![inhibit the enzyme
dihydrofolate reductase, which
catalyzes the conversion of
dihydrofolate into
tetrahydrofolate, the active
form of folic acid
Naproxen (nsaids)
Naproxen blocks arachidonate
binding to competitively inhibit
both cyclooxygenase (COX)
isoenzymes, COX-1 and COX-
2, resulting in analgesic and
anti-inflammatory effects.
Flanax, Inza,
Maxidol, Nalgesin,
Naposin, Naprelan,
Naprogesic,
Naprosyn, Narocin,
Pronaxen, Proxen,
and Soproxen.
FDA-approved for
treating acute gout,
ankylosing
spondylitis, bursitis,
osteoarthritis,
tendonitis,
rheumatoid arthritis,
pain, and primary
dysmenorrhea
Naproxen is
known to cause
renal failure by
renal papillary
necrosis
Omeprazole(Proton
Pump Inhibitors)
It is a substituted benzimidazole
that belongs to the antisecretory
class of compounds. It inhibits
the parietal cell H+ / K+ ATP
pump, the final step of acid
production.
Prilosec and
Losec,pan-d
Recent clinical
data point to a
subtle nephrotoxic
effect of
Omeprazole, but
the cellular and
molecular
mechanisms are
unknown
Penicillin-
G(Antibacterial
antibiotics)
Penicillin inhibits the cross-
linking of peptidoglycan in the
cell wall.[5] The catalyst for
this reaction is penicillin-
binding proteins, such as the
enzyme DD-transpeptidase.
Bicillin LA,
Permapen
Renal toxicity,
Pravastatin(Antihyper
lipidemic agents)
Competitive
hydroxymethylglutaryl
coenzyme-A (HMG Co-A)
reductase inhibitors.
Pravachol®
Reduced the rate
of kidney
function loss
Rifampicin(Antibacte
rial agents)
Rifampin produces bactericidal
antimicrobial activity by
inhibiting DNA-dependent
RNA polymerase (RNAP)
either by sterically blocking the
Rifadin® and
Rimactane®.
Reversible renal
failure](https://image.slidesharecdn.com/diriwithcommercialavailableagents-240305184630-4e3fdb77/75/DIRI-with-commercial-available-agents-pdf-17-2048.jpg)






