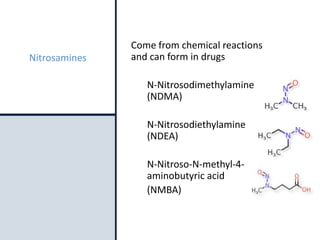
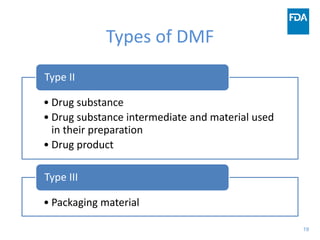
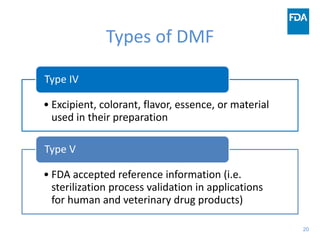
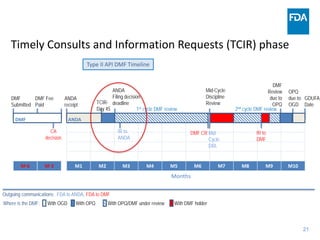
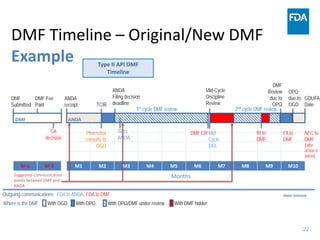
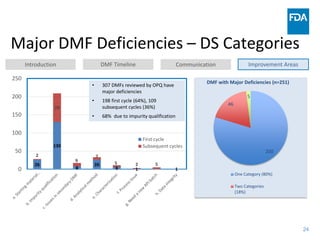
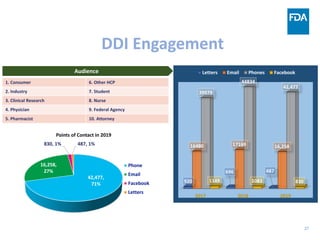
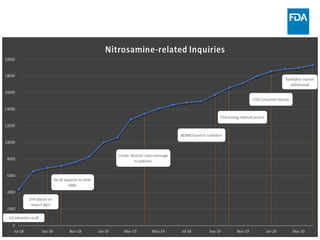
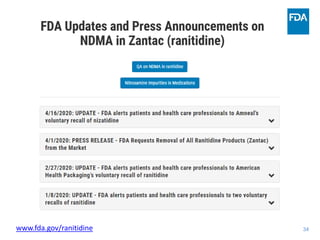
The document discusses nitrosamine impurities that have been found in certain drug products and FDA's role in addressing this issue. It provides background on nitrosamines, describes drug classes affected like ARBs and ranitidine, and outlines resources for obtaining more information such as FDA's website. It also discusses FDA's focus on ensuring pharmaceutical quality and protecting public health through monitoring drug supply chains and collaborating globally.