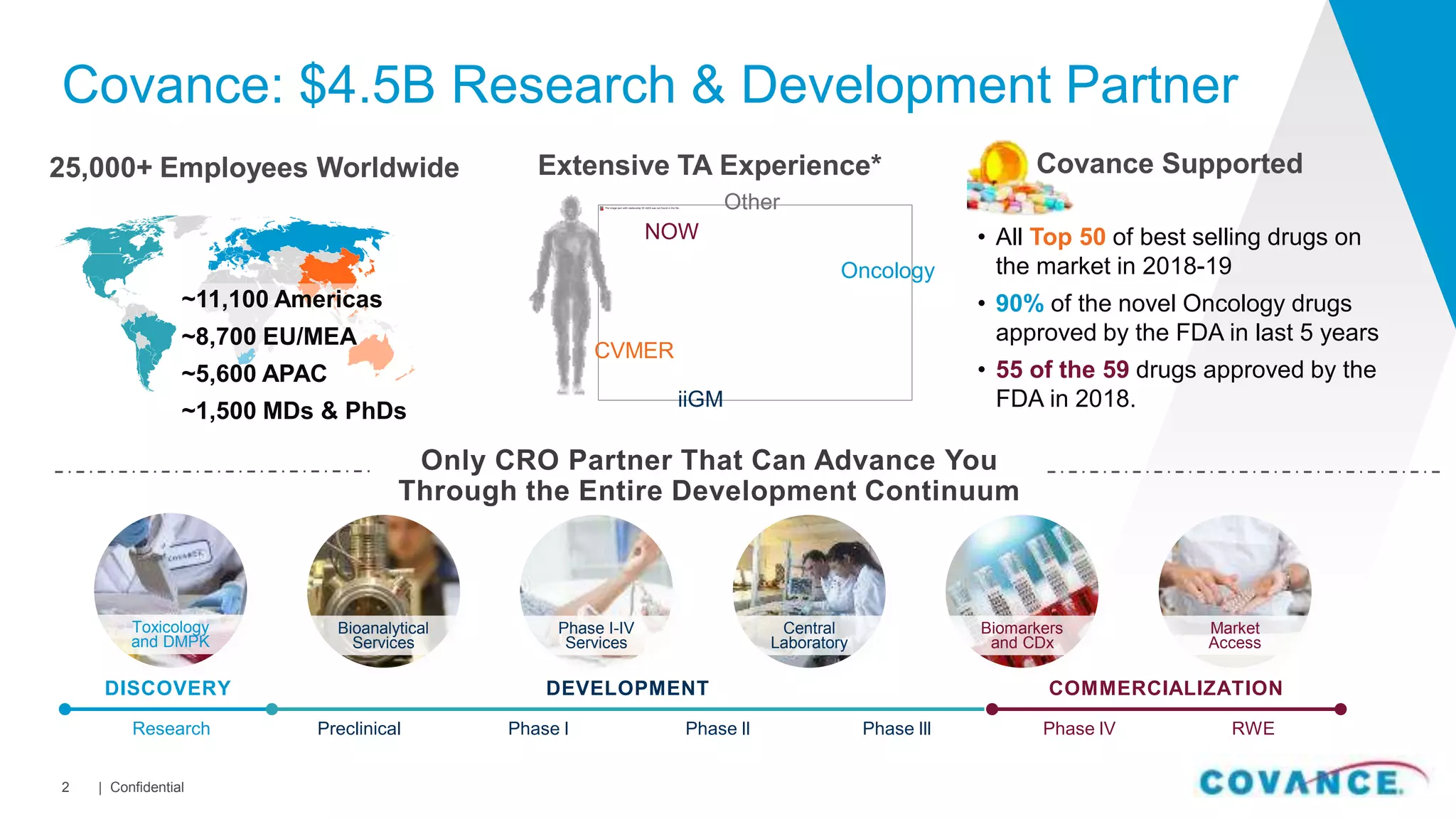
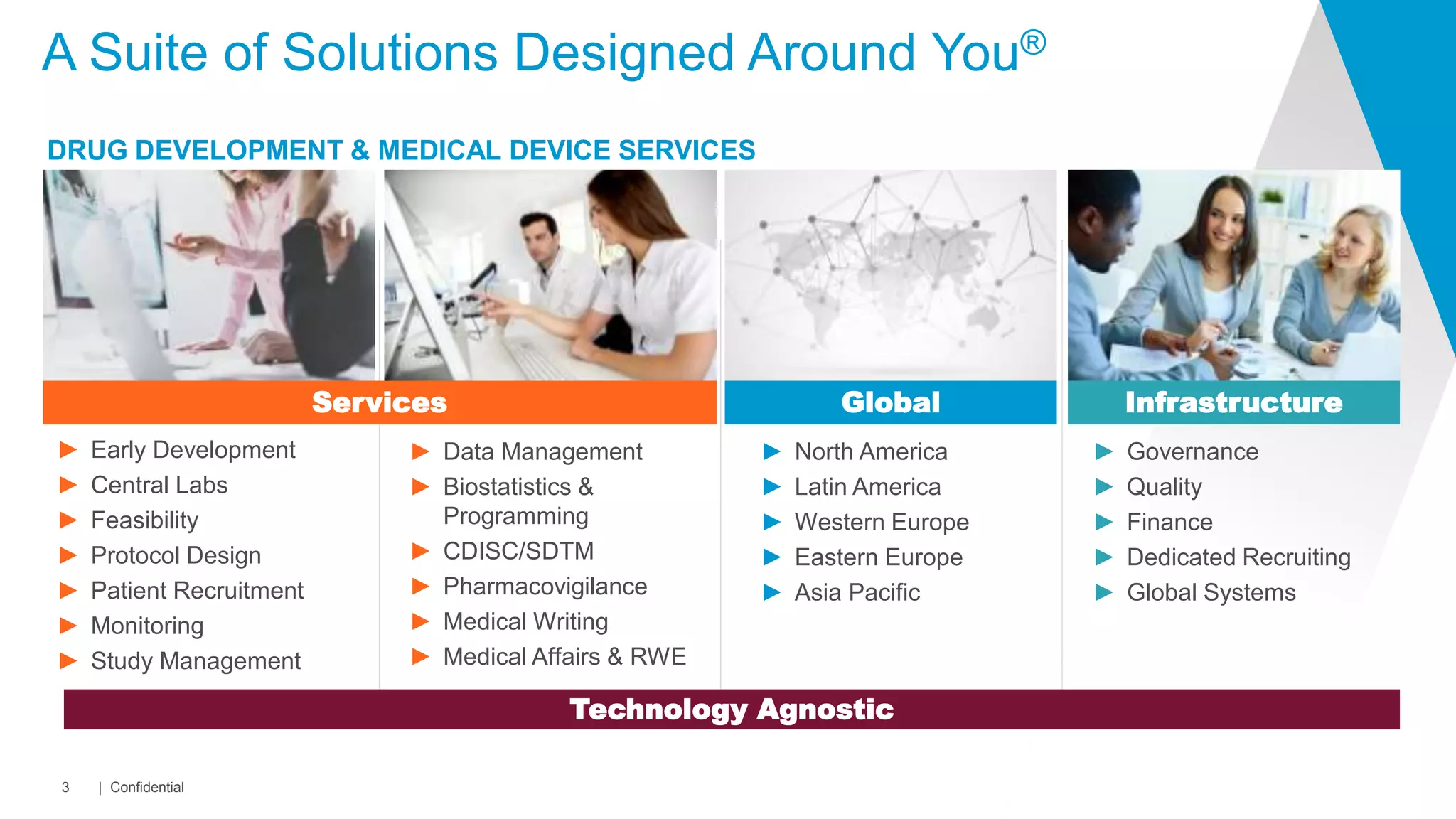
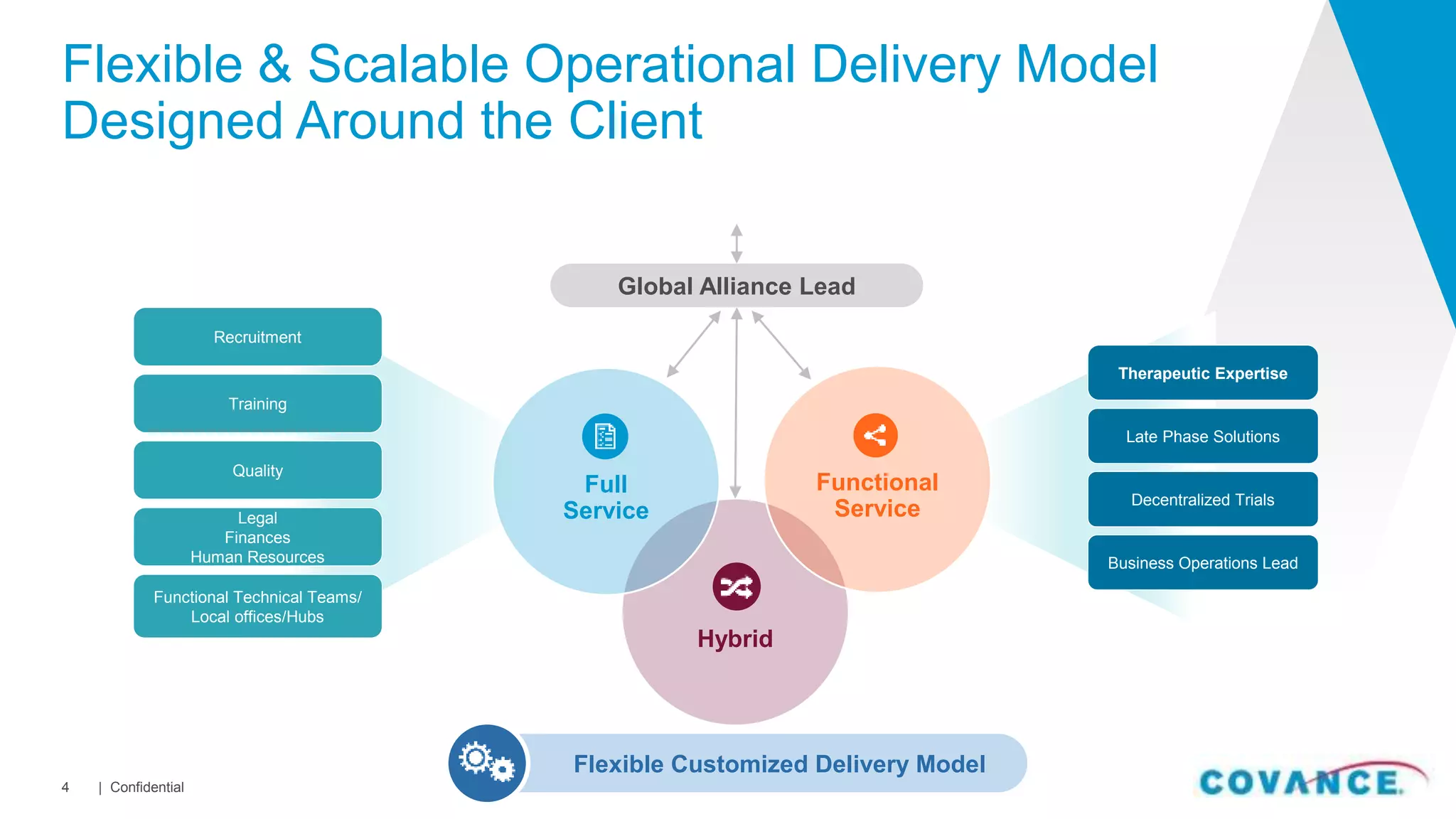
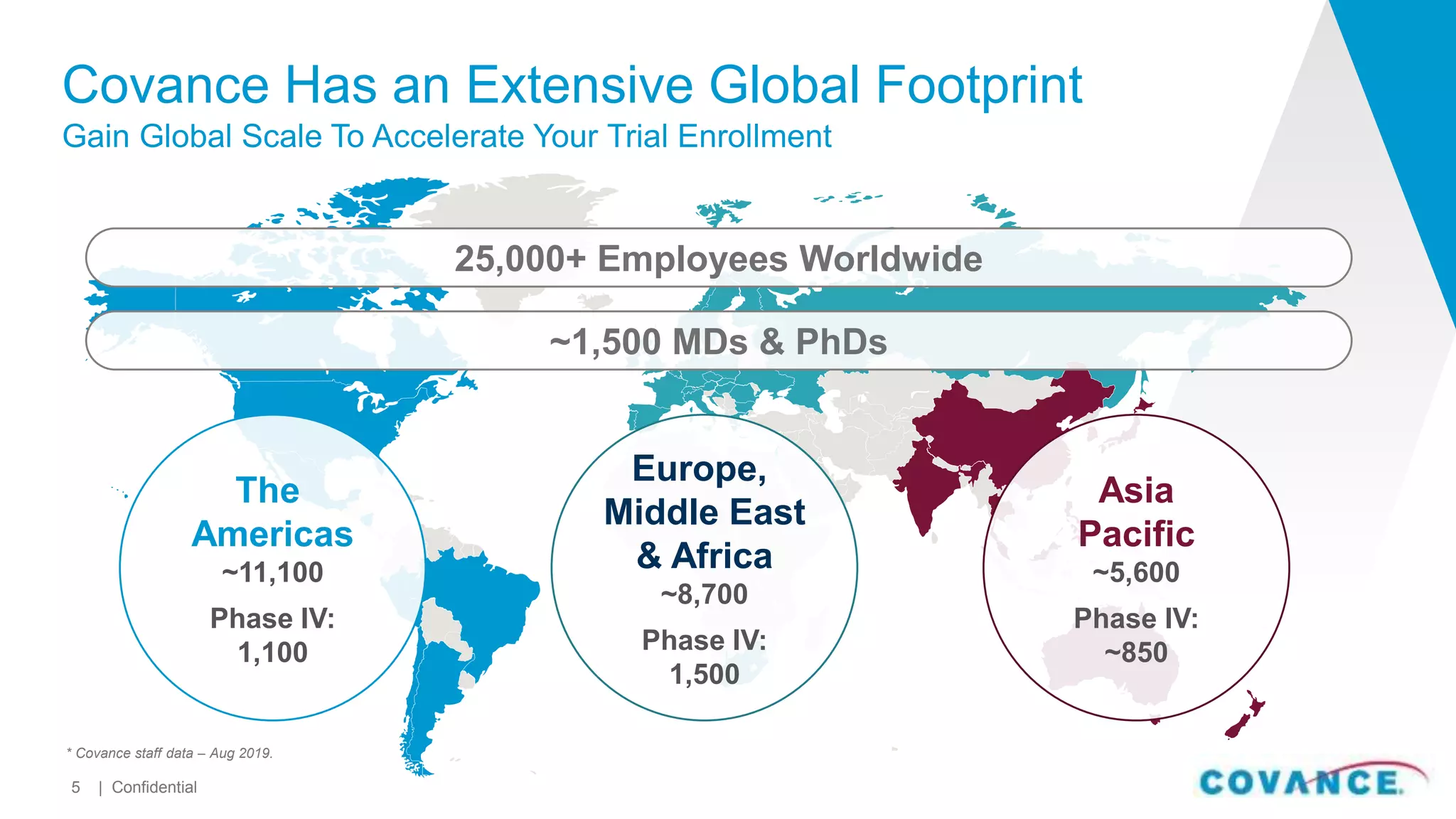
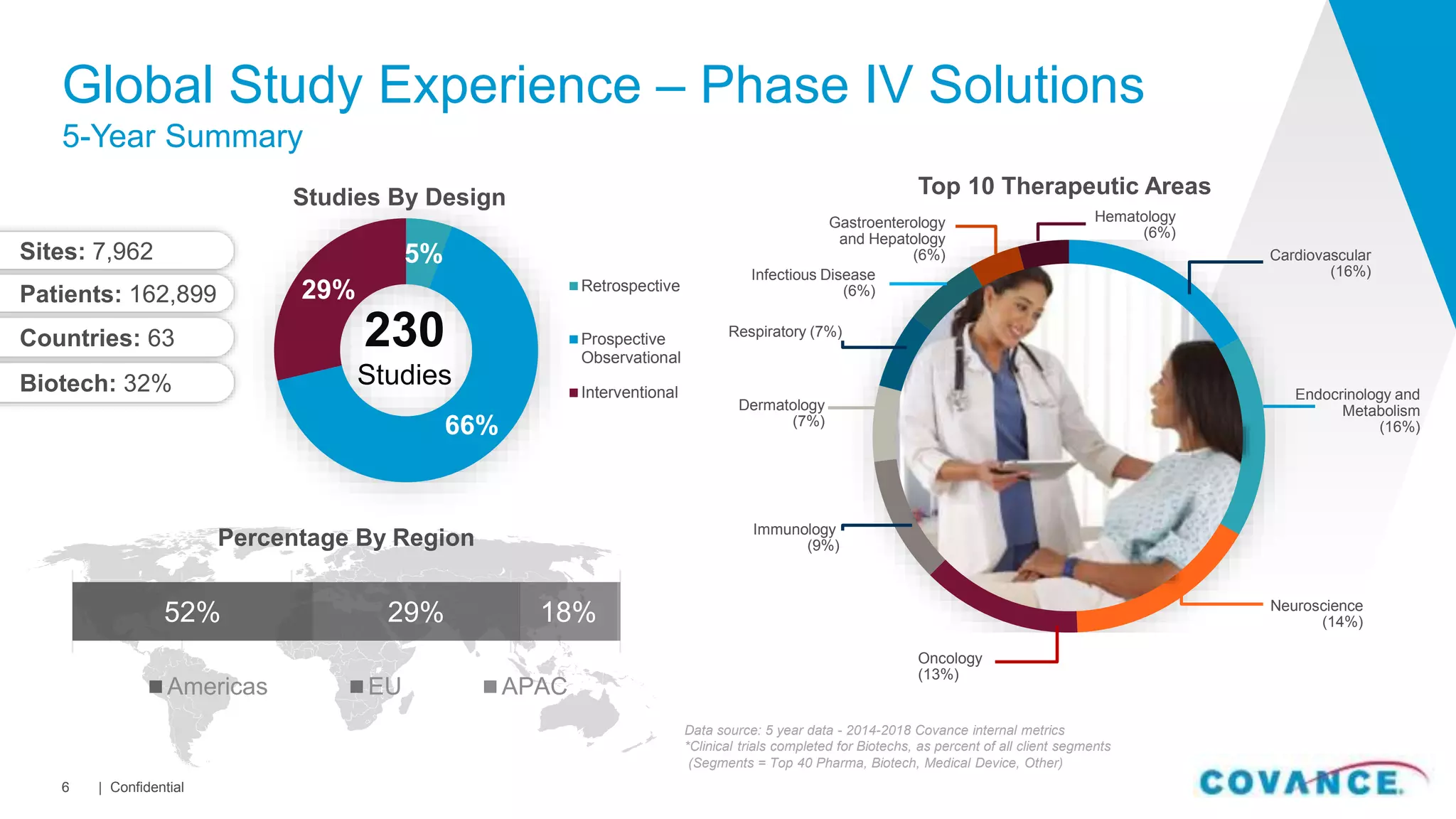
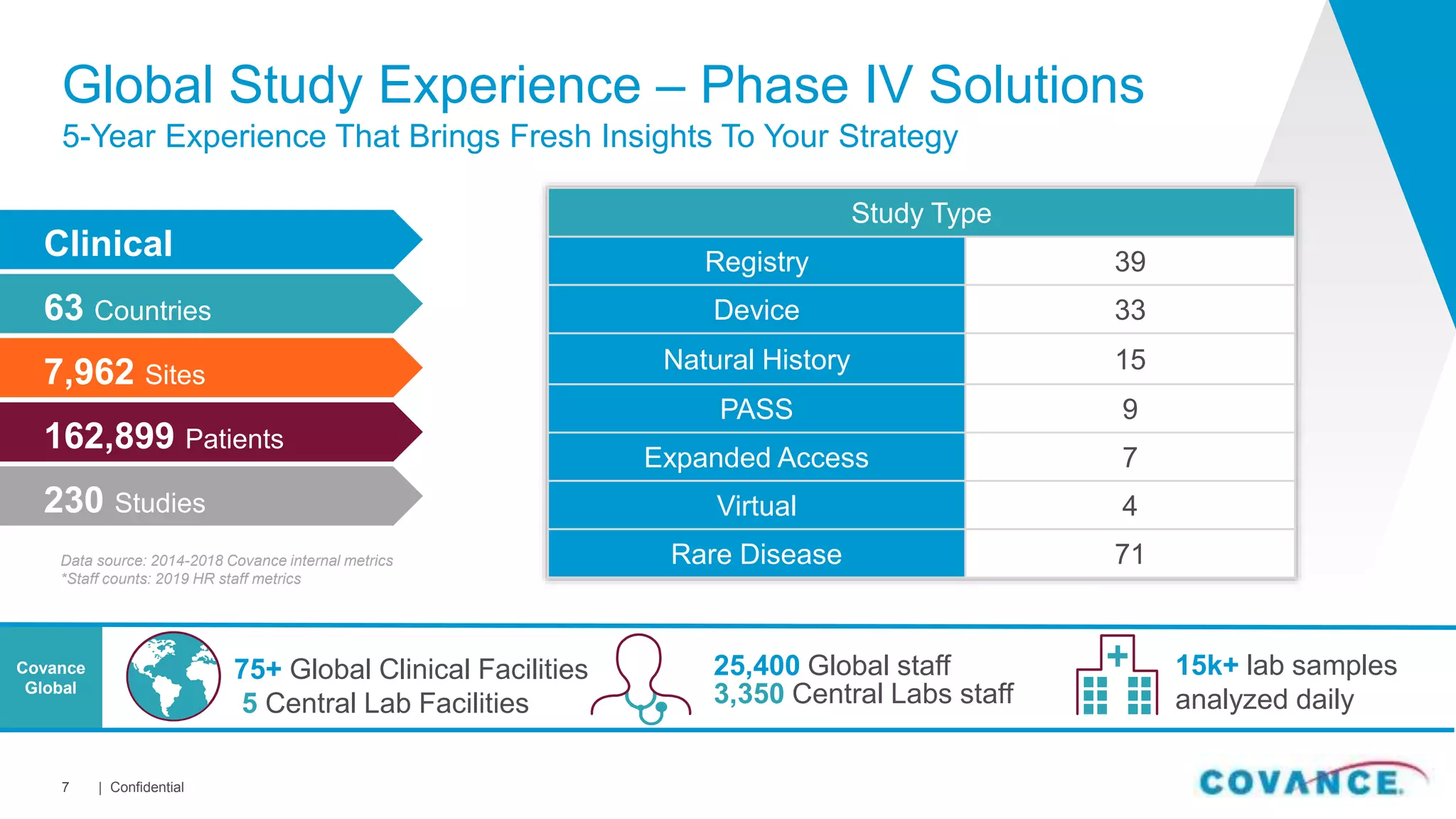
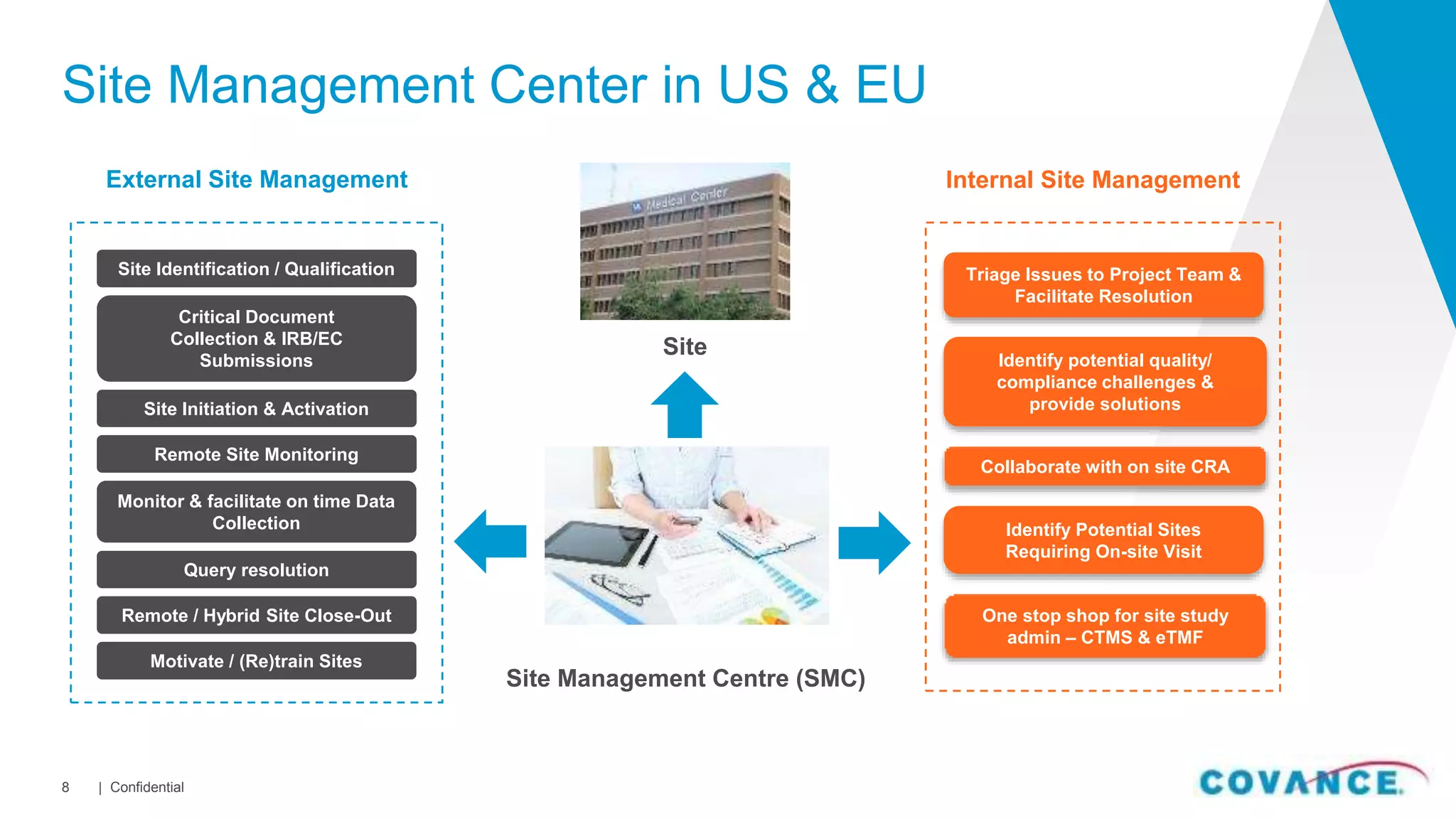
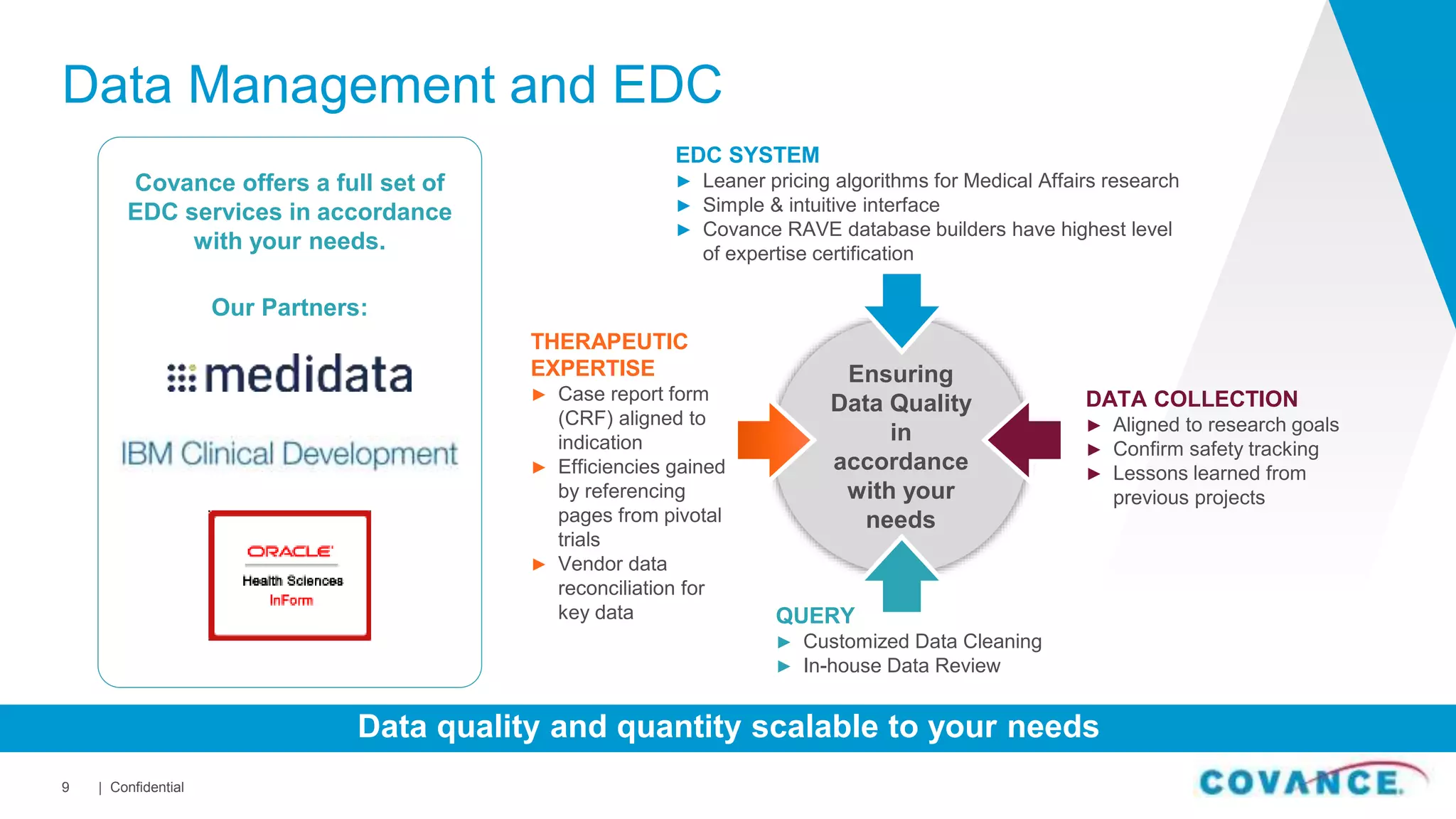
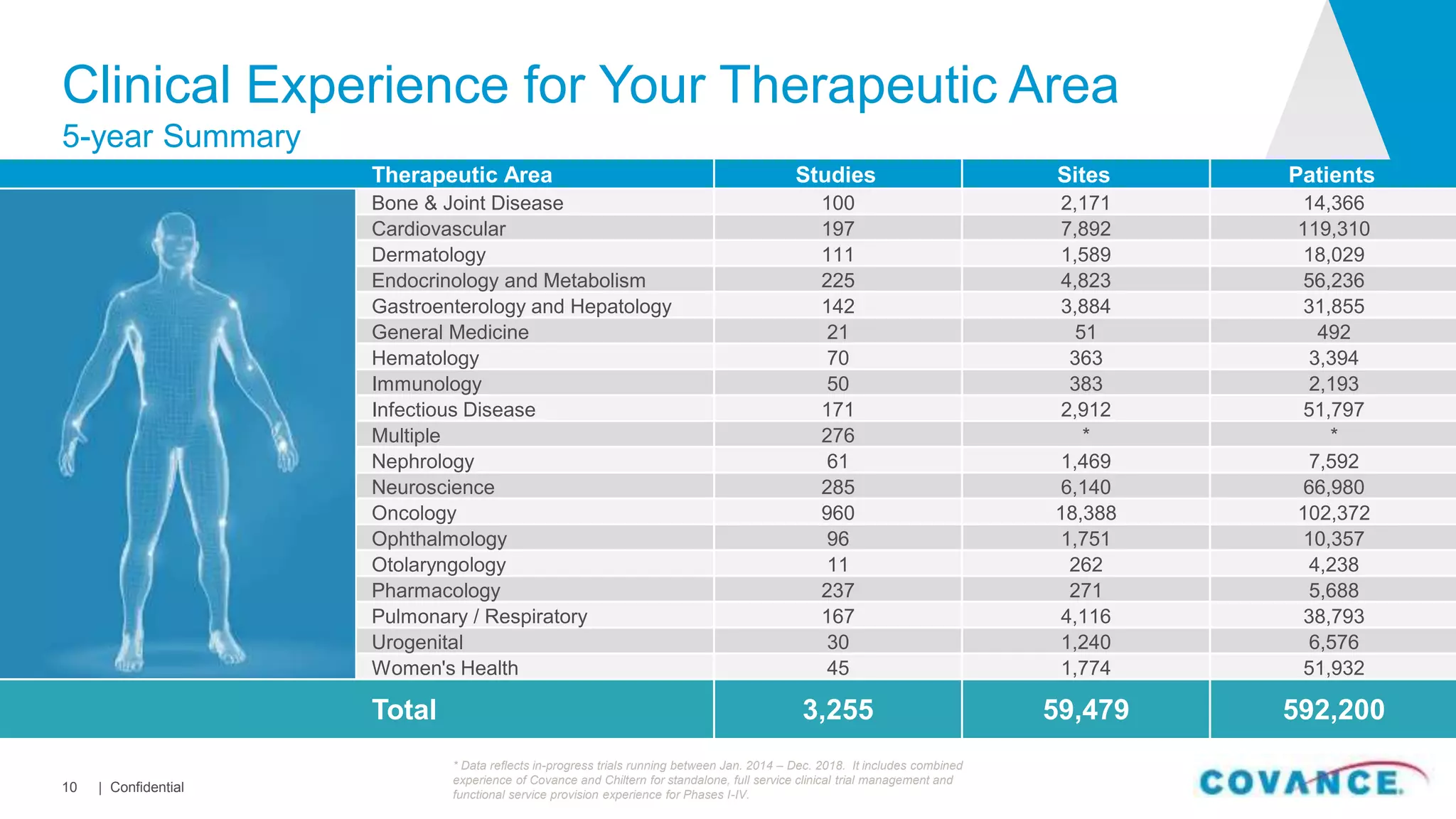
This document summarizes Covance's capabilities in drug development and clinical research. Covance has over 25,000 employees worldwide, including over 1,500 medical doctors and PhDs. It has supported over 90% of novel oncology drugs approved by the FDA in the last 5 years and 55 of 59 drugs approved by the FDA in 2018. Covance offers services across the drug development lifecycle from discovery to commercialization.