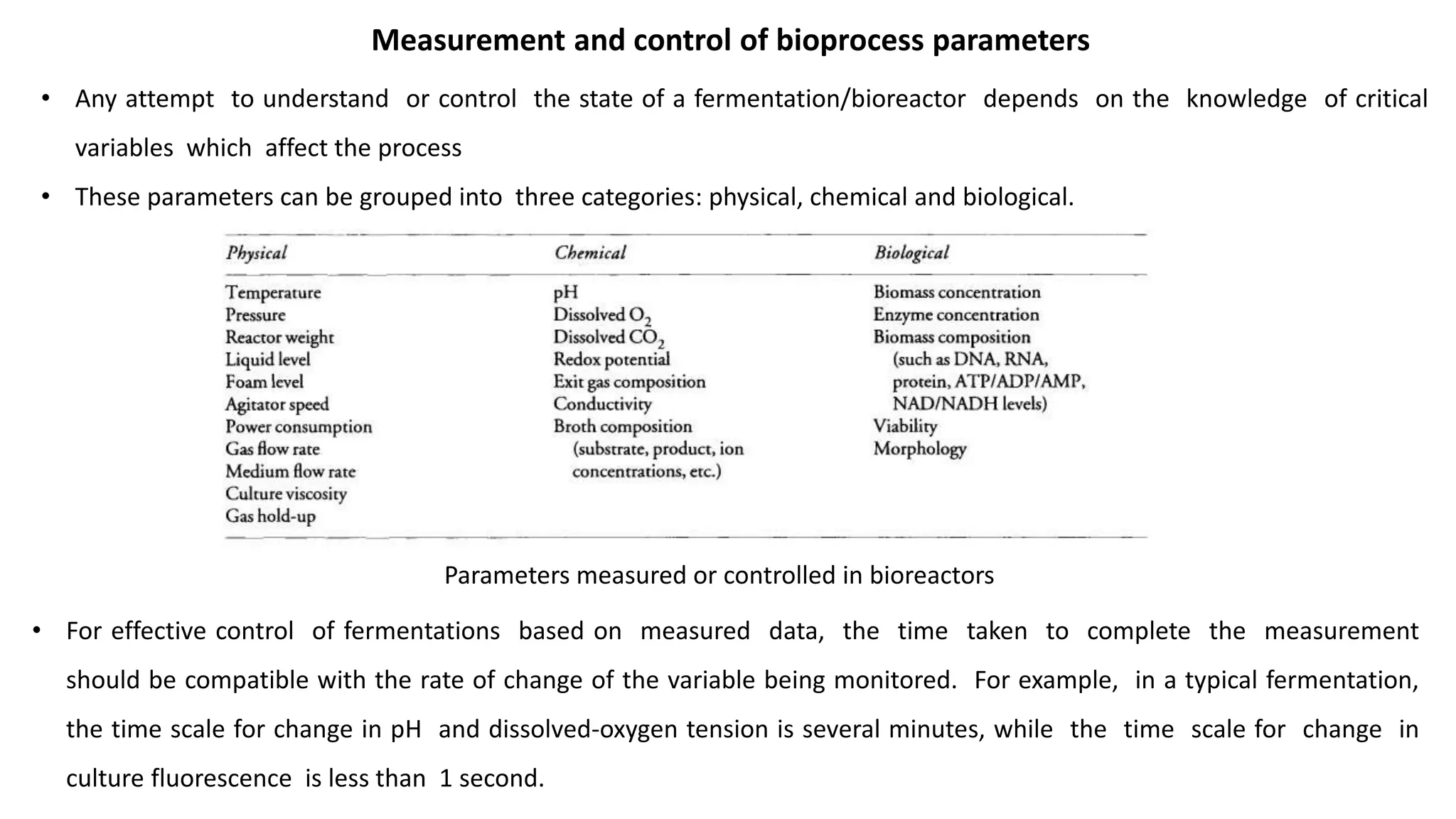
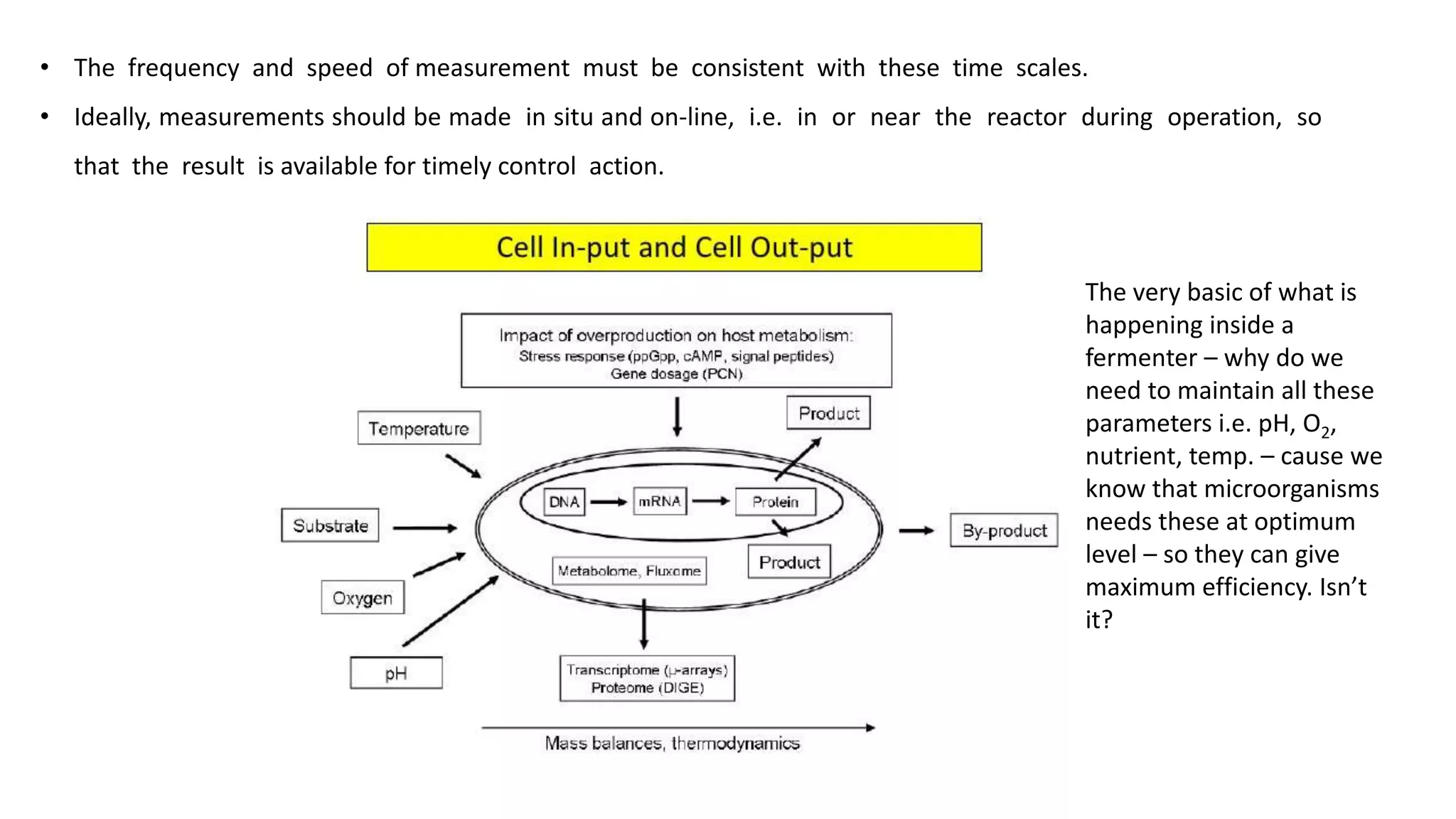
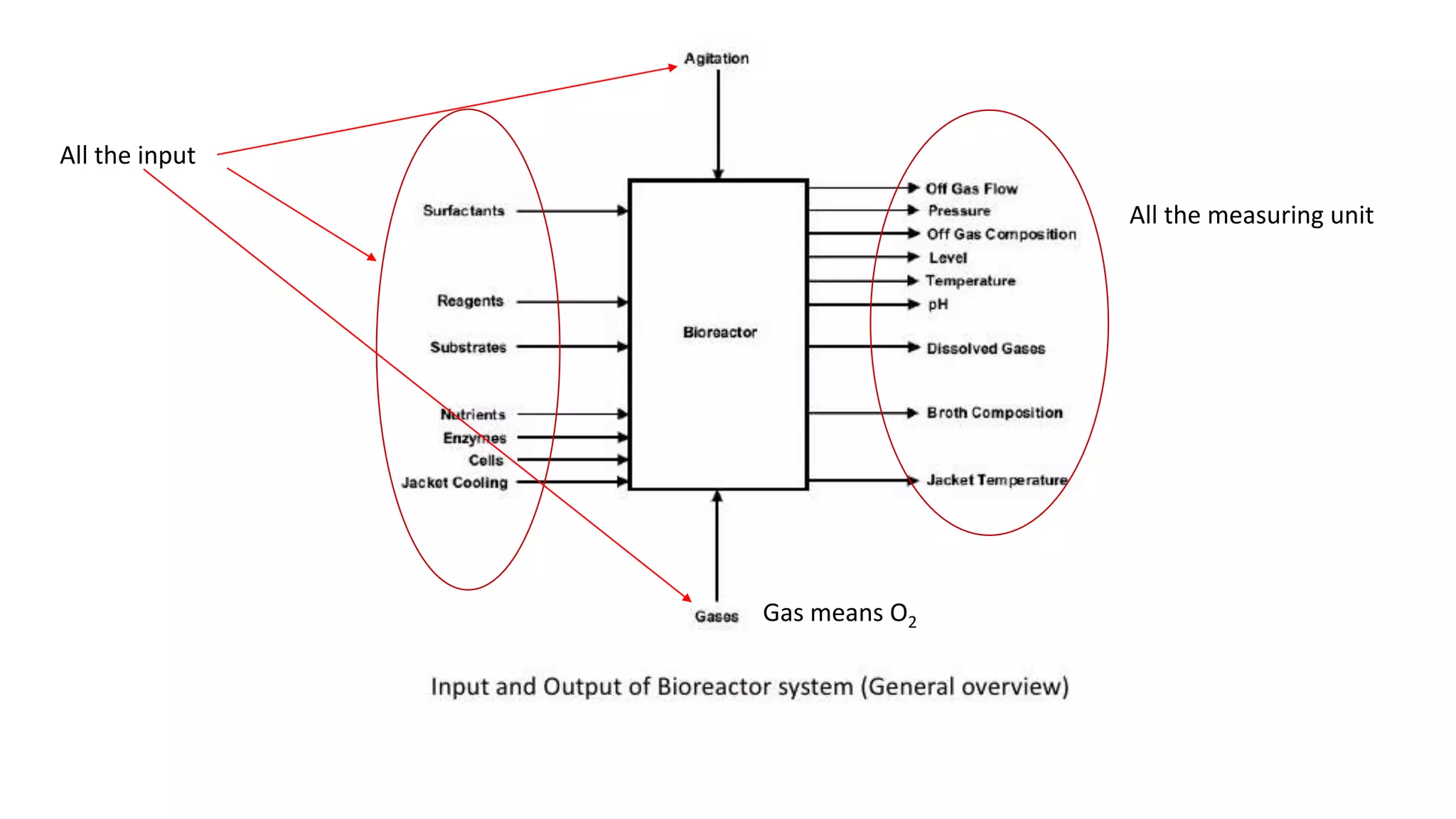
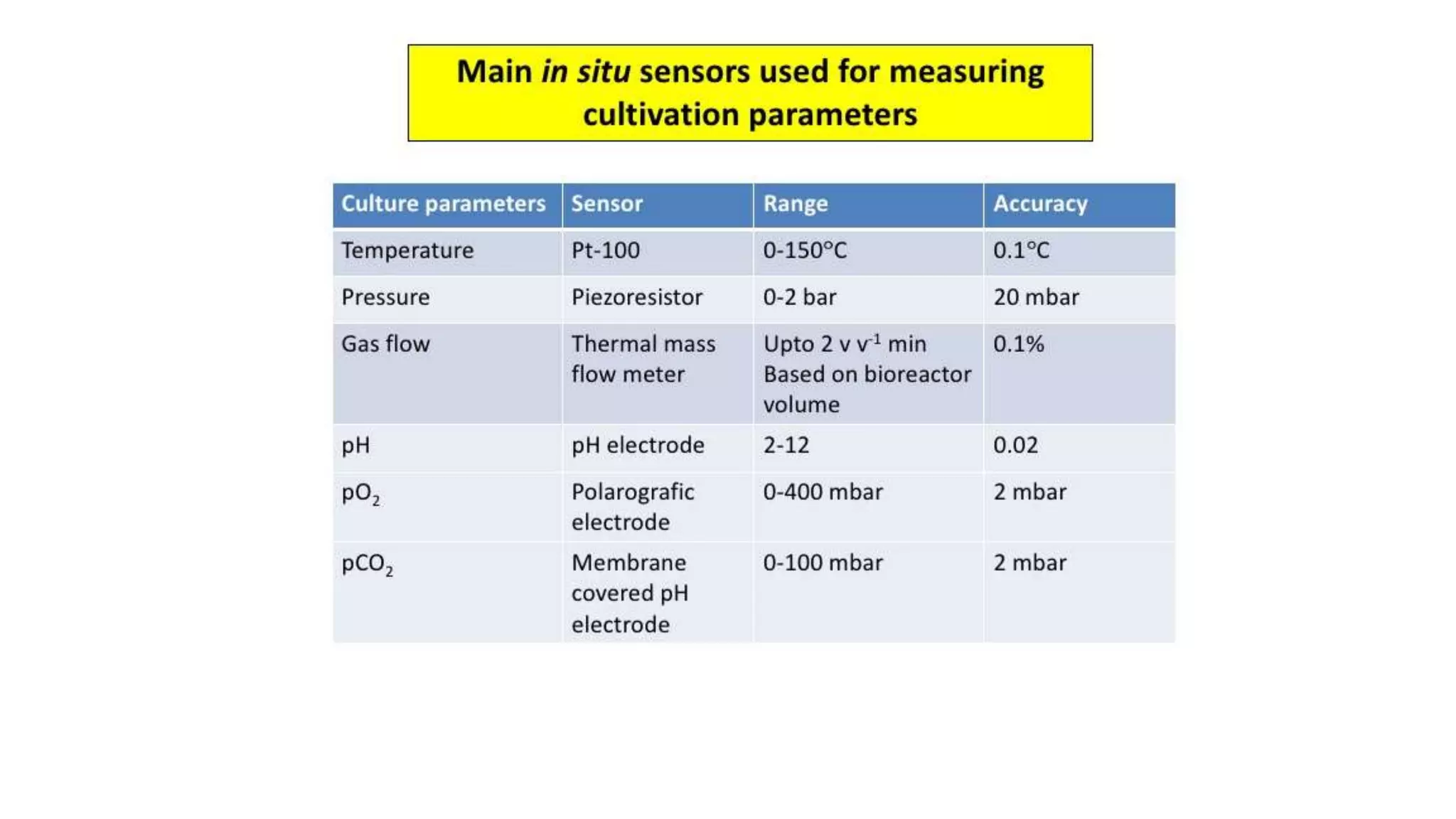
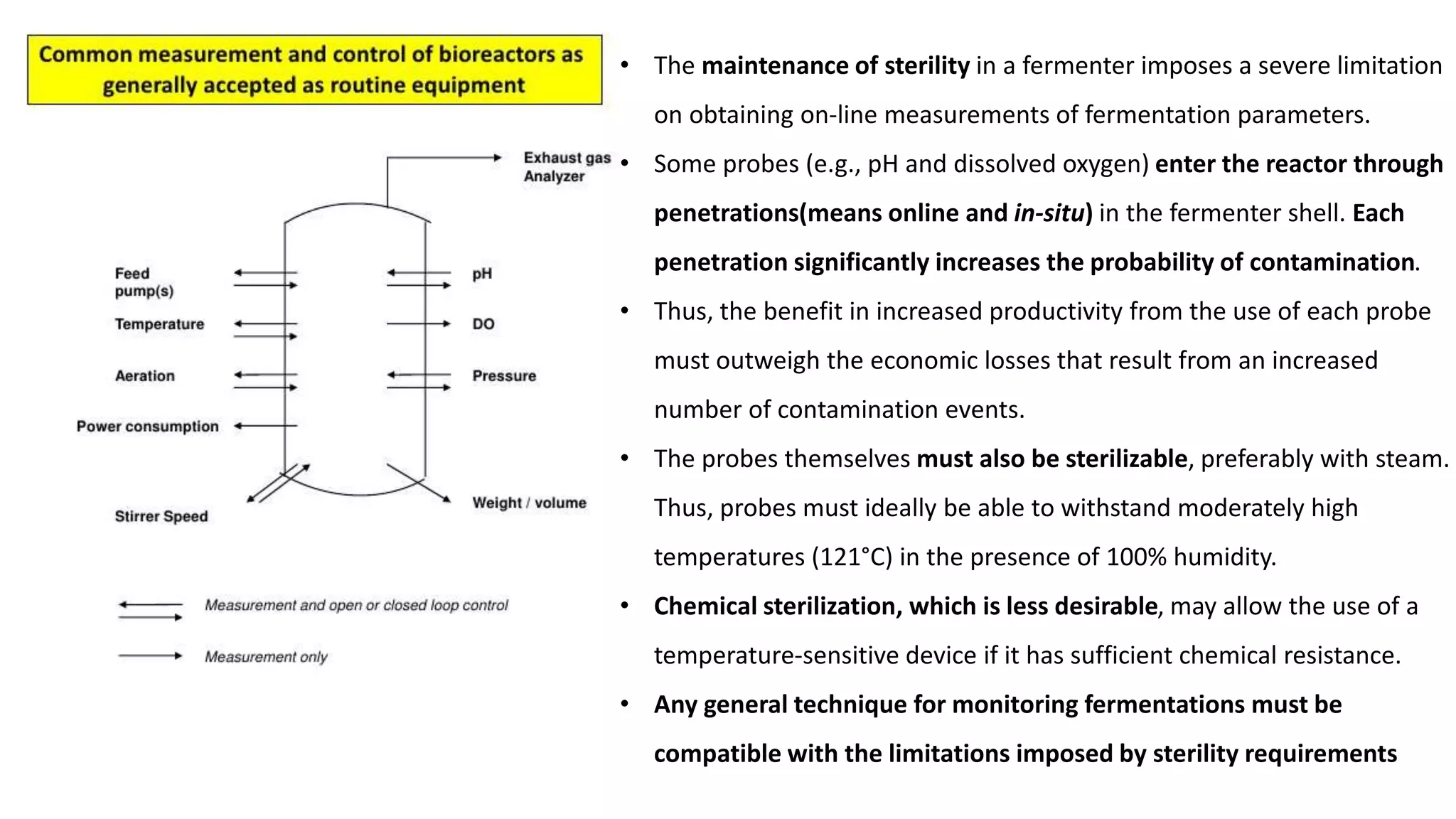
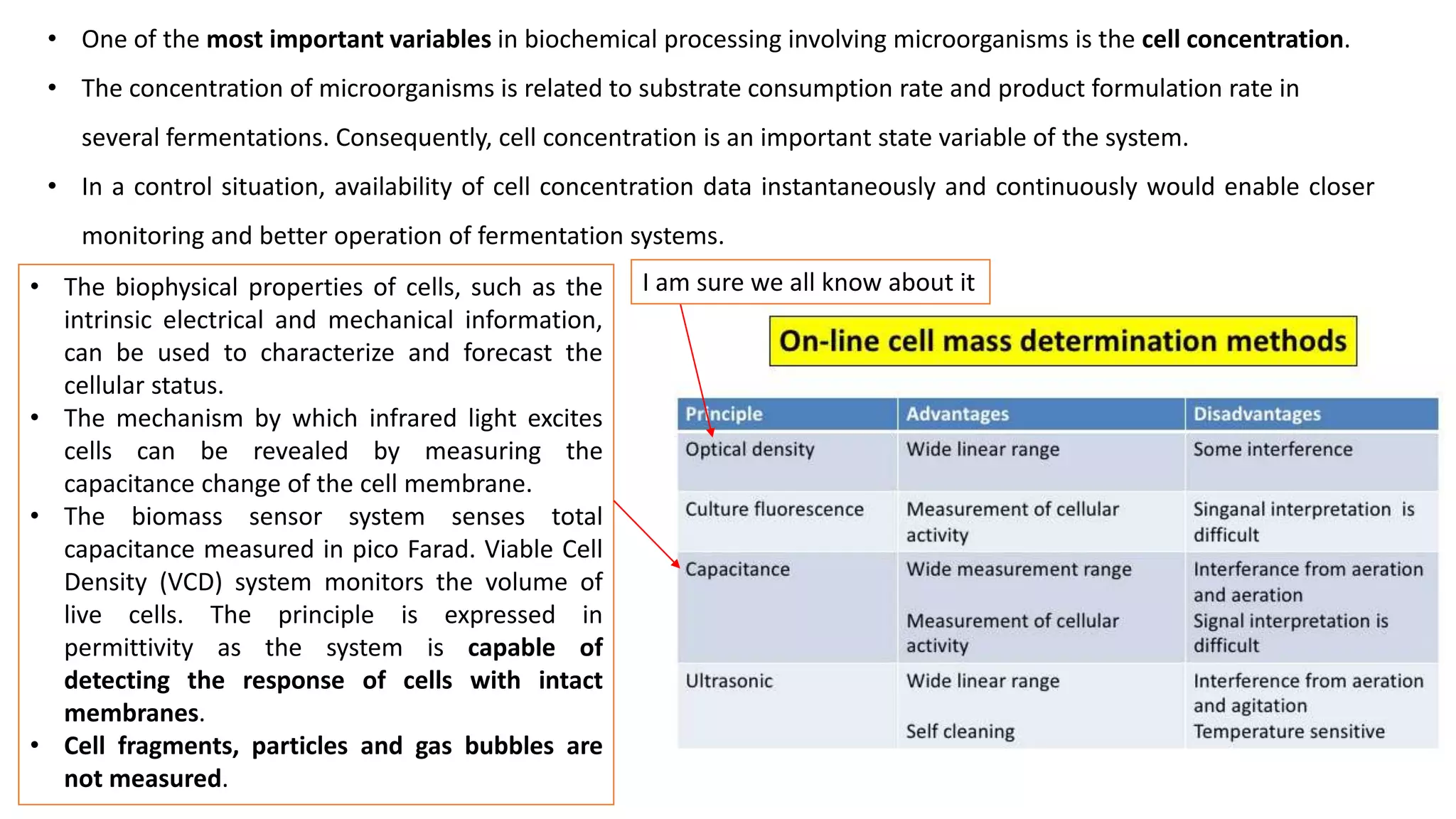
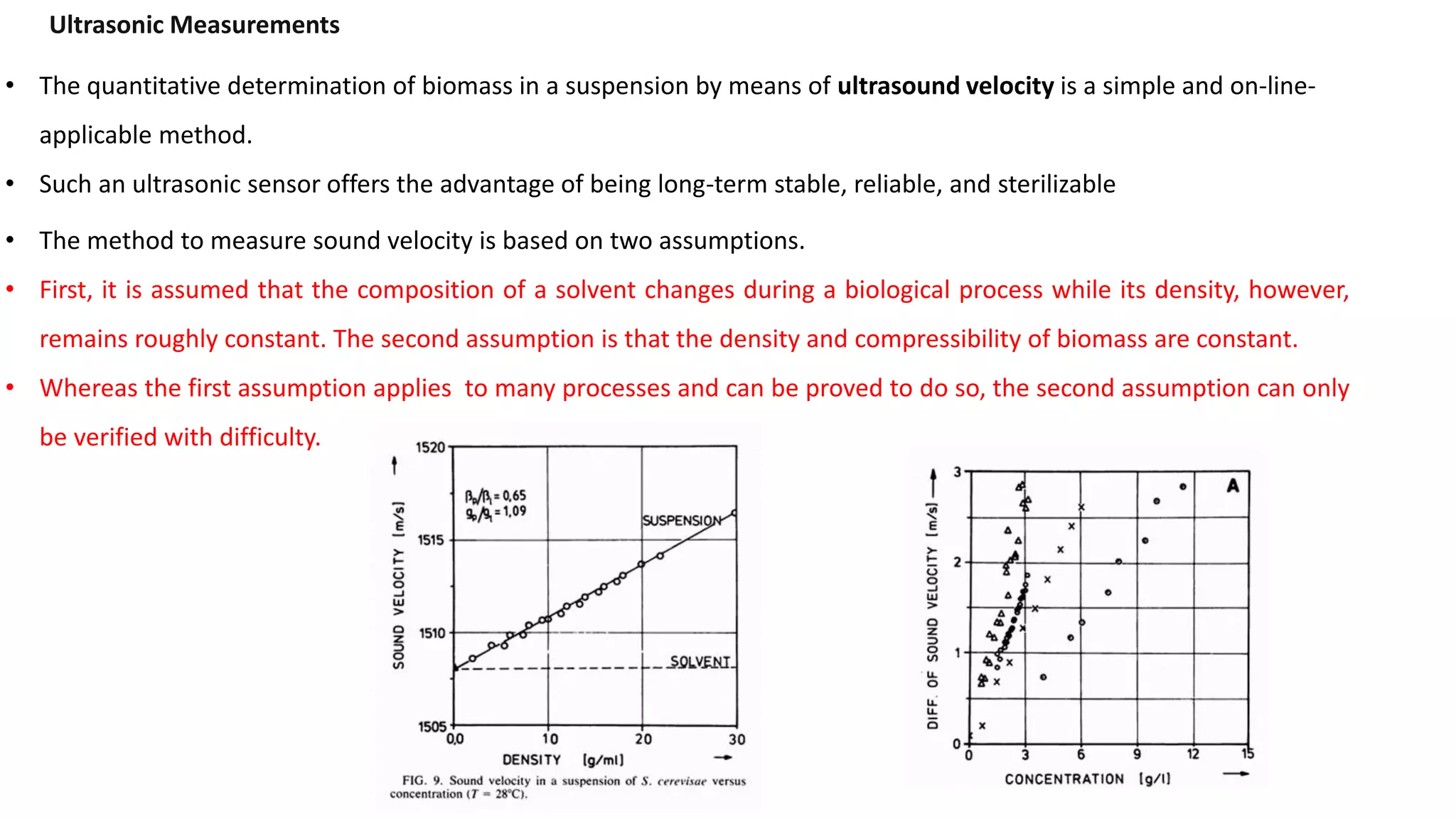
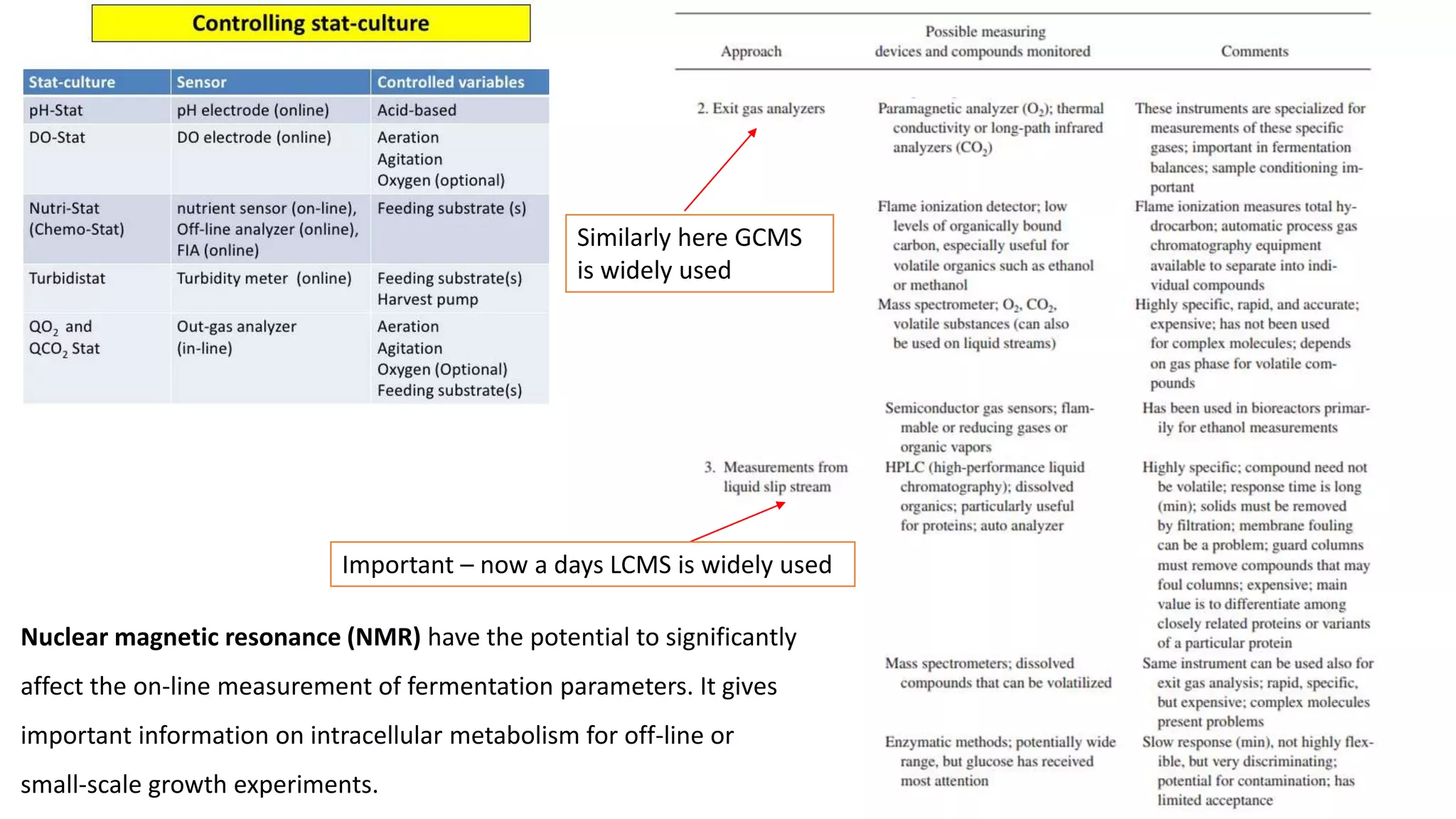
This document discusses parameters that must be measured or controlled in bioreactors to effectively monitor and regulate fermentation processes. These include physical parameters like pH, dissolved oxygen, and temperature, as well as biological parameters like cell concentration. Several challenges are outlined for obtaining online, in-situ measurements of these parameters, such as maintaining sterility, probe stability over long fermentations, and avoiding fouling. Specific techniques are also described for measuring parameters like culture fluorescence, ultrasonic biomass detection, and NMR analysis of intracellular metabolism.