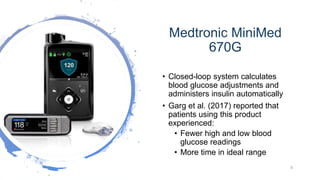
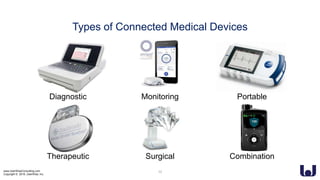
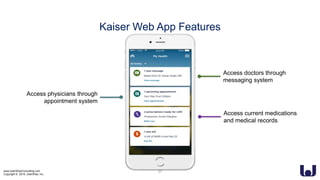
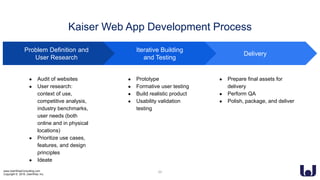
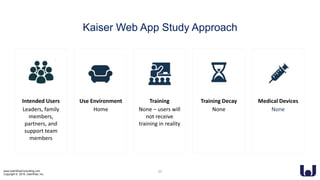
This document discusses enhancing the user experience of connected medical devices. It provides an overview of connected medical devices and FDA regulations. Examples of connected devices like an autoinjector app and insulin pump are described. Considerations for the design and evaluation of connected devices through usability testing are discussed. Human factors and risk analysis are important to consider for safety. The document concludes that connected devices require examining how information is exchanged and ensuring usability, reliability, safety and security through the design process.