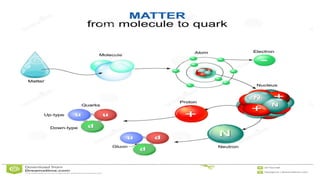
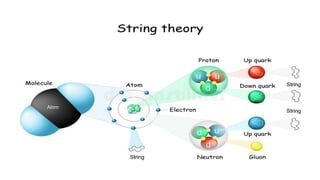
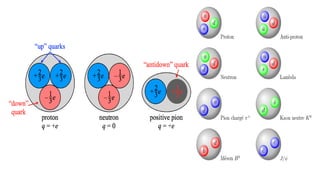
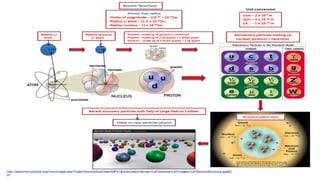
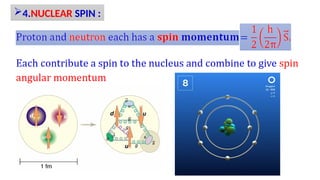
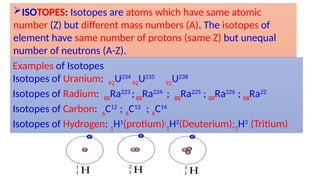
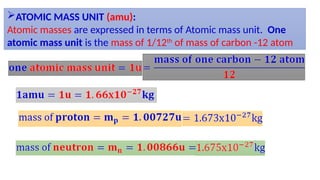
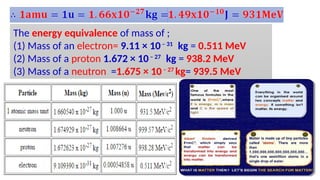
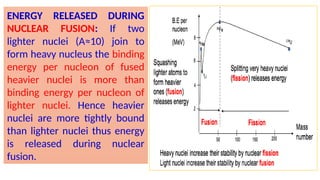
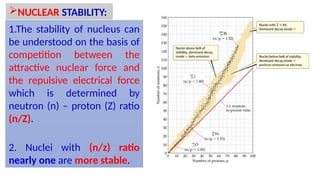
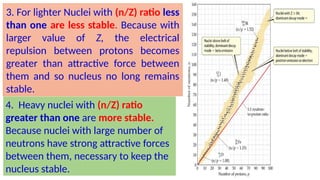
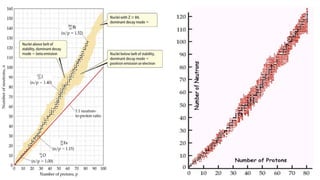
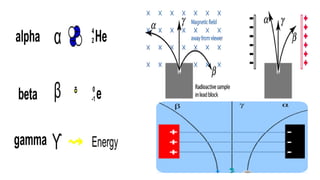
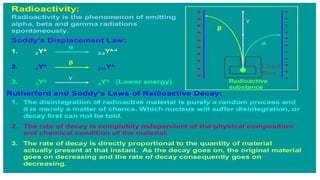
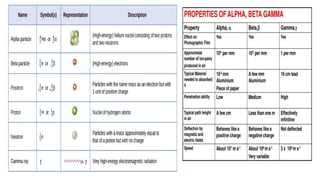
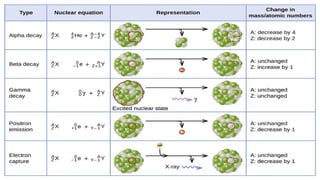
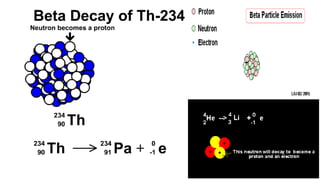
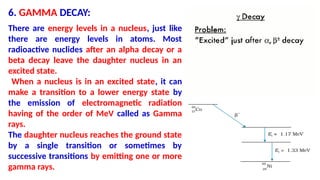
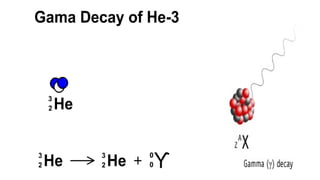
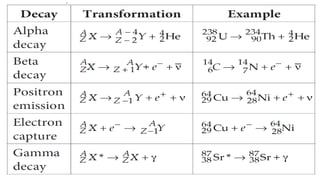
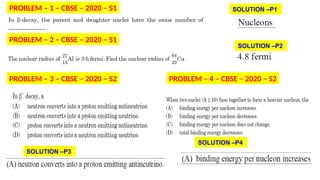
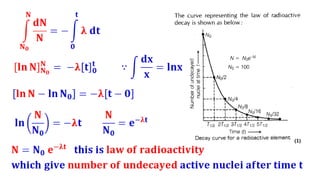
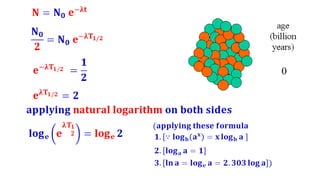
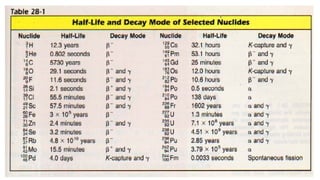
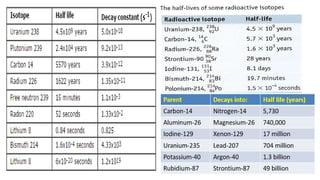
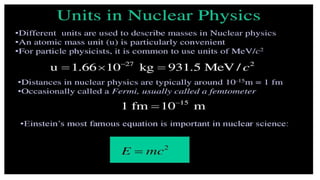
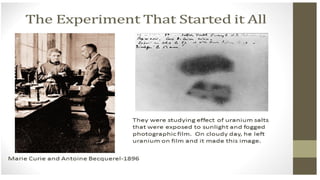
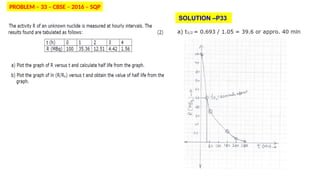
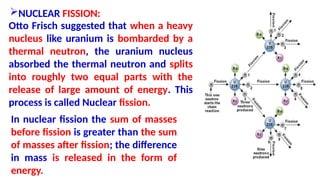
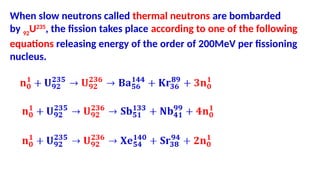
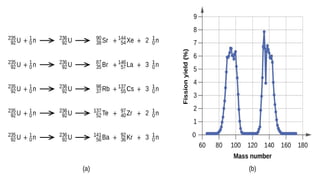
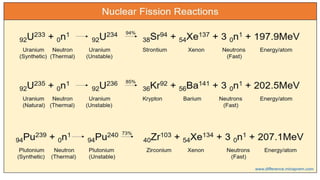
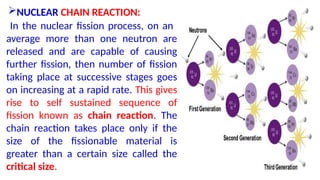
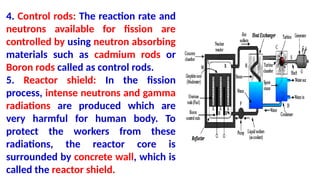
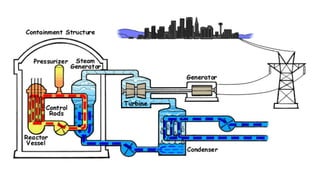
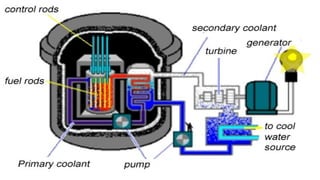
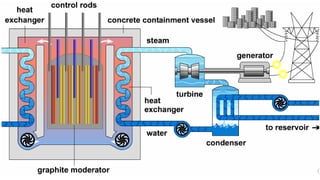
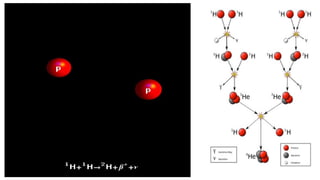
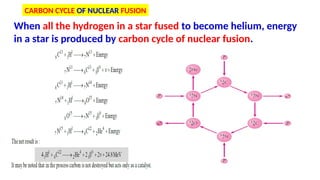
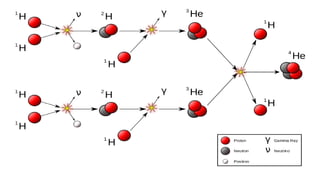
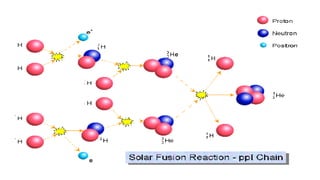
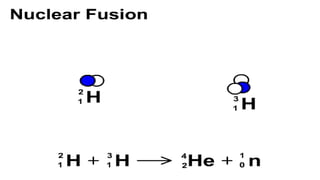
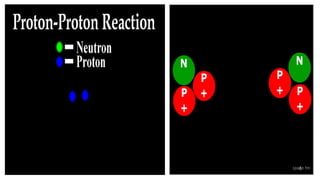
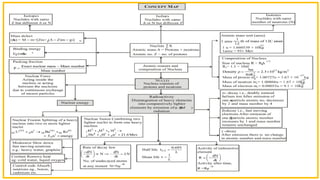
The Atoms chapter in Class 12 Physics plays a crucial role in understanding the microscopic structure of matter and the quantum nature of energy. This chapter marks a transition from classical physics to modern physics, explaining how atomic models evolved and how quantization of energy revolutionized our understanding of atoms.
Introduction to Atoms
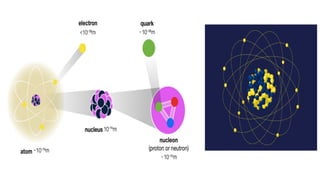
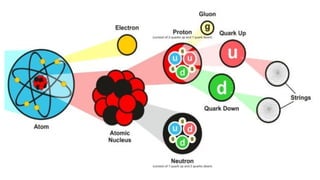
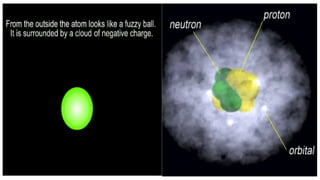
An atom is the smallest unit of matter that retains the chemical properties of an element. This chapter explores how scientists gradually uncovered the internal structure of atoms through experiments and theoretical models, leading to the development of quantum concepts.
Early Atomic Models
The chapter begins with historical atomic models:
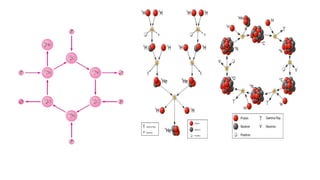
Thomson’s model (plum pudding model)
Rutherford’s nuclear model
Rutherford’s gold foil experiment revealed:
The presence of a small, positively charged nucleus
Most of the atom is empty space
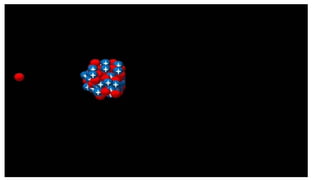
However, classical physics could not explain atomic stability, leading to further developments.
Bohr’s Model of Hydrogen Atom
A major highlight of the chapter, Bohr’s atomic model, successfully explained the stability and emission spectra of hydrogen.
Key postulates:
Electrons move in discrete circular orbits
Only certain orbits are allowed with quantized angular momentum
Energy is emitted or absorbed when electrons transition between orbits
Energy Levels and Orbits
The chapter explains:
Quantized energy levels
Radius of nth orbit
Velocity of electron in an orbit
Important formulas include:
𝐸
𝑛
=
−
13.6
𝑛
2
eV
E
n
=−
n
2
13.6
eV
𝑟
𝑛
=
𝑛
2
𝑎
0
r
n
=n
2
a
0
Hydrogen Spectrum
Atoms emit or absorb energy in discrete wavelengths, forming line spectra.
Key spectral series:
Lyman series (UV region)
Balmer series (visible region)
Paschen, Brackett, and Pfund series (IR region)
The Rydberg formula explains spectral lines:
1
𝜆
=
𝑅
(
1
𝑛
1
2
−
1
𝑛
2
2
)
λ
1
=R(
n
1
2
1
−
n
2
2
1
)
Limitations of Bohr’s Model
While successful for hydrogen-like atoms, Bohr’s model could not explain:
Multi-electron atoms
Zeeman effect
Fine structure of spectral lines
These limitations paved the way for quantum mechanics.
Important Topics Covered
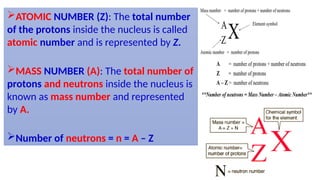
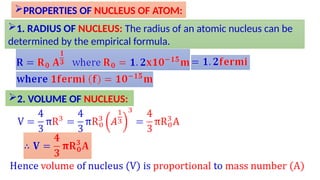
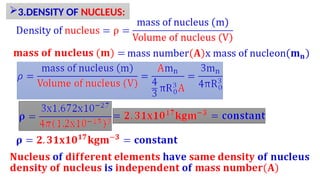
Atomic number and mass number
Excitation and ionization energy
Ground state and excited states
Emission and absorption of radiation
Applications of Atomic Theory
Spectroscopy
Atomic clocks
Lasers
Identification of elements
Astrophysics and stellar analysis
Why This Chapter Is Important
Core chapter of modern physics
High exam weightage
Strong numerical problem-solving content
Foundation for quantum mechanics and nuclear physics
Conclusion
The Atoms chapter provides deep insight into the structure and behavior of matter at the atomic level. By introducing energy quantization and atomic spectra, it lays the groundwork for understanding advanced topics in physics and modern technology.