Embed presentation
Download to read offline
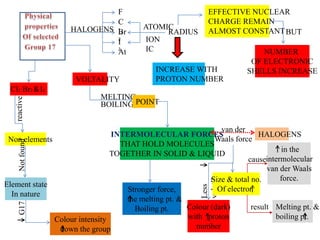

The document discusses how properties of halogens change down the periodic table from fluorine to iodine. The atomic and ionic radii increase with proton number, while the number of electronic shells remains the same. The intermolecular forces holding molecules together, such as van der Waals forces, get weaker with increasing size and number of electrons. As a result, melting and boiling points decrease as volatility increases from fluorine to iodine.
