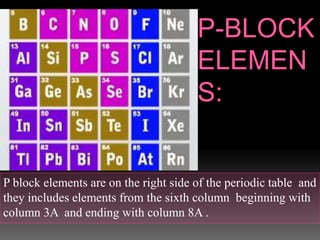
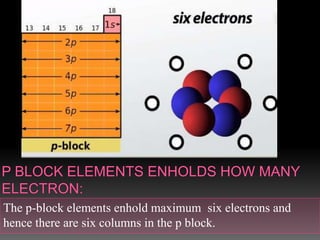
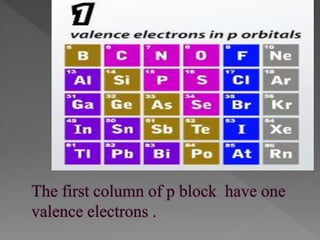
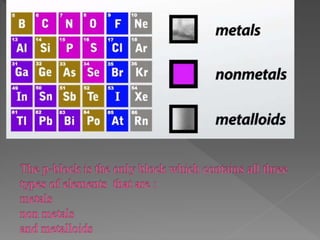
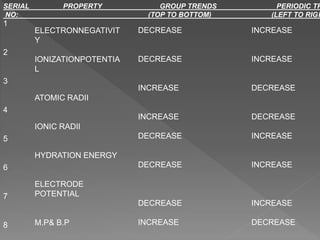
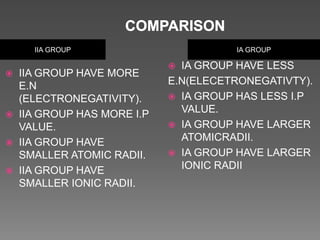
The document discusses two topics: an introduction to p-block elements and periodic trends in groups and periods. It defines p-block elements as those found on the right side of the periodic table from column 3A to 8A, whose valence electrons are in p orbitals. It lists some major periodic trends like electronegativity, ionization energy, atomic radius, and melting point that decrease from top to bottom in a group but increase from left to right across a period. It also compares trends in groups IA and IIA.