This document summarizes key concepts about cell signaling pathways. It discusses:
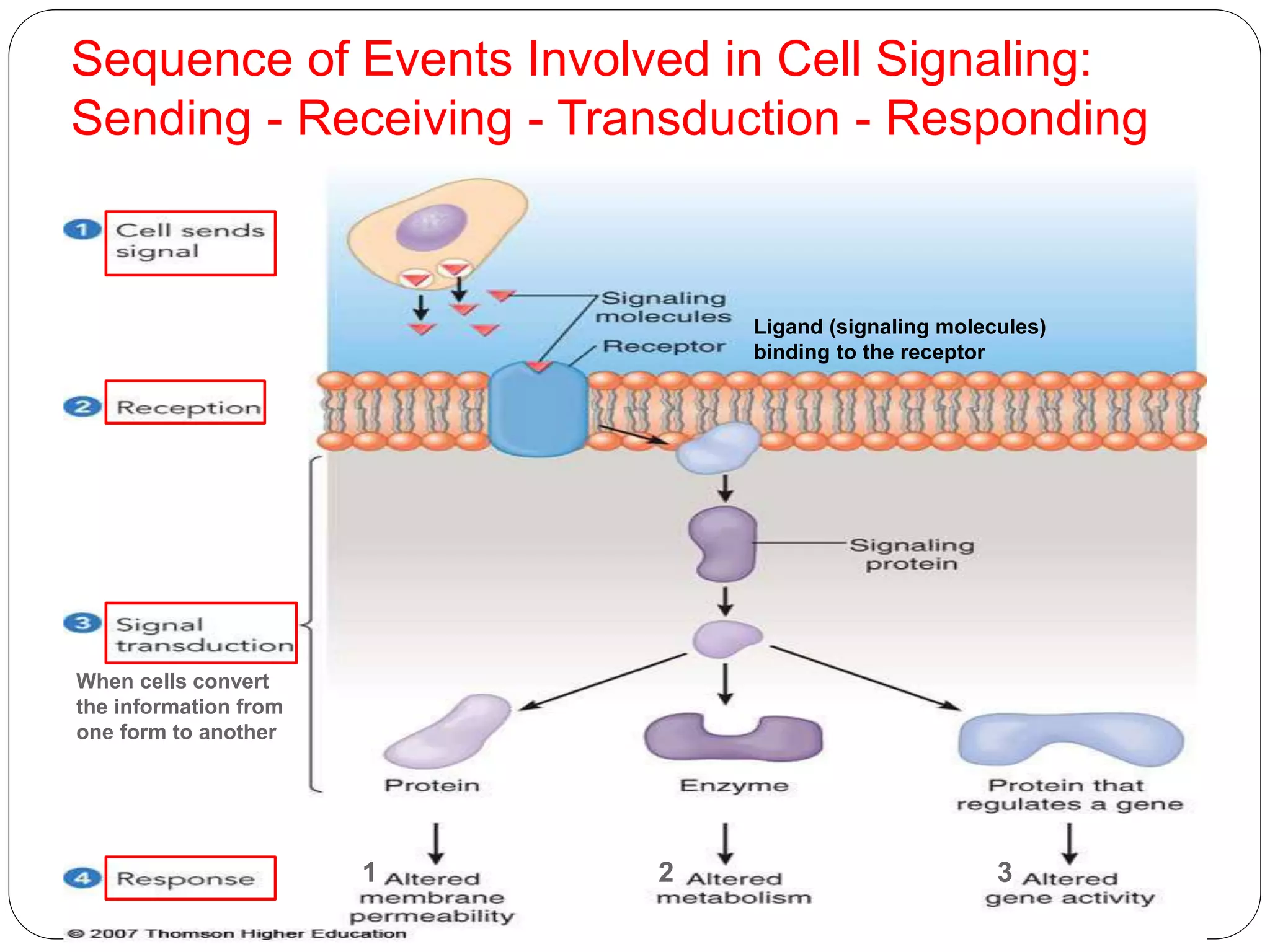
1) Signal transduction pathways translate ligand-receptor interactions into biochemical changes in cells. Upstream components are near the receptor, downstream components near effector molecules.
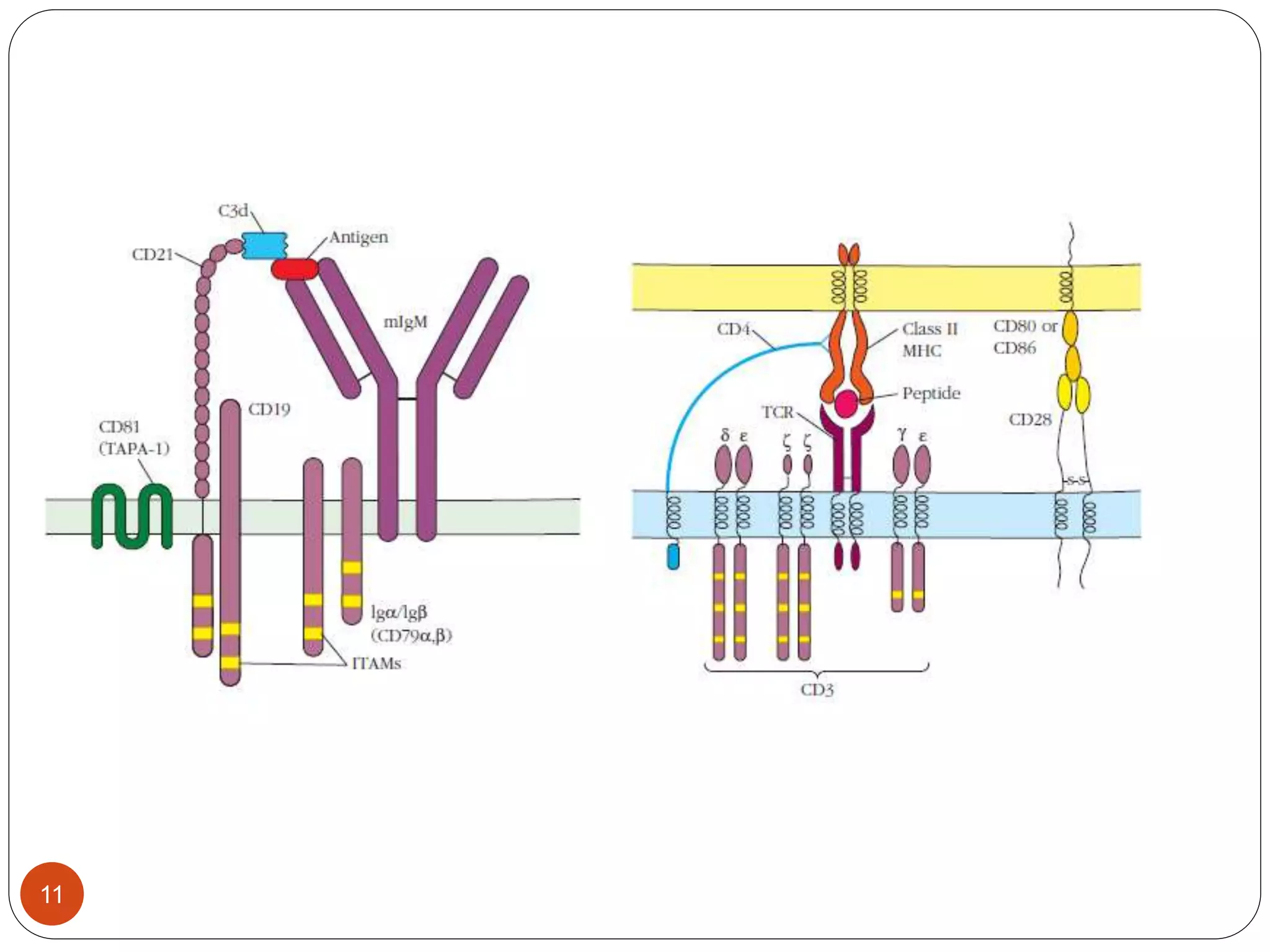
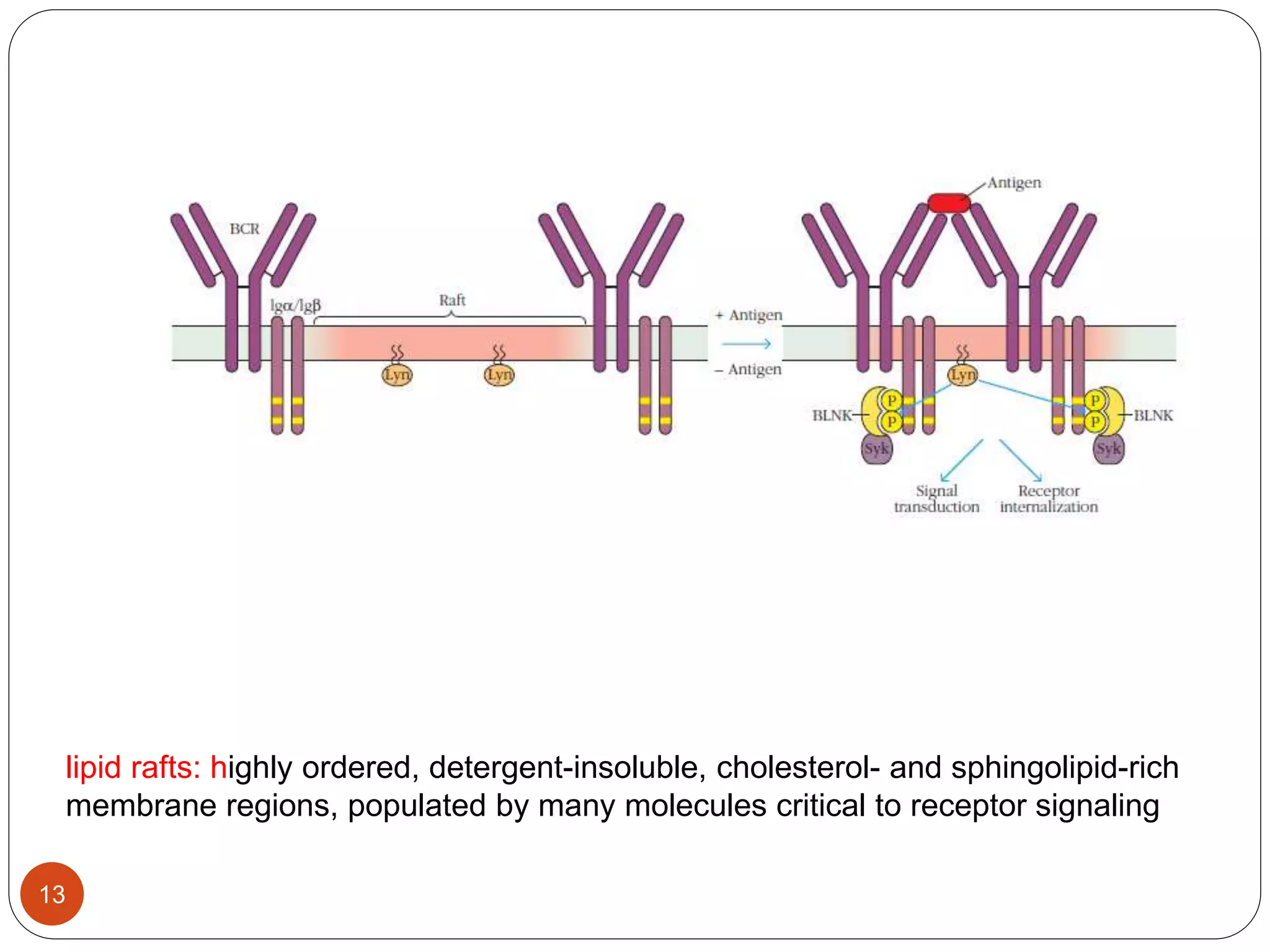
2) Ligand binding often induces receptor dimerization or polymerization via conformational changes. Receptor-associated molecules like ITAMs transmit signals into cells.
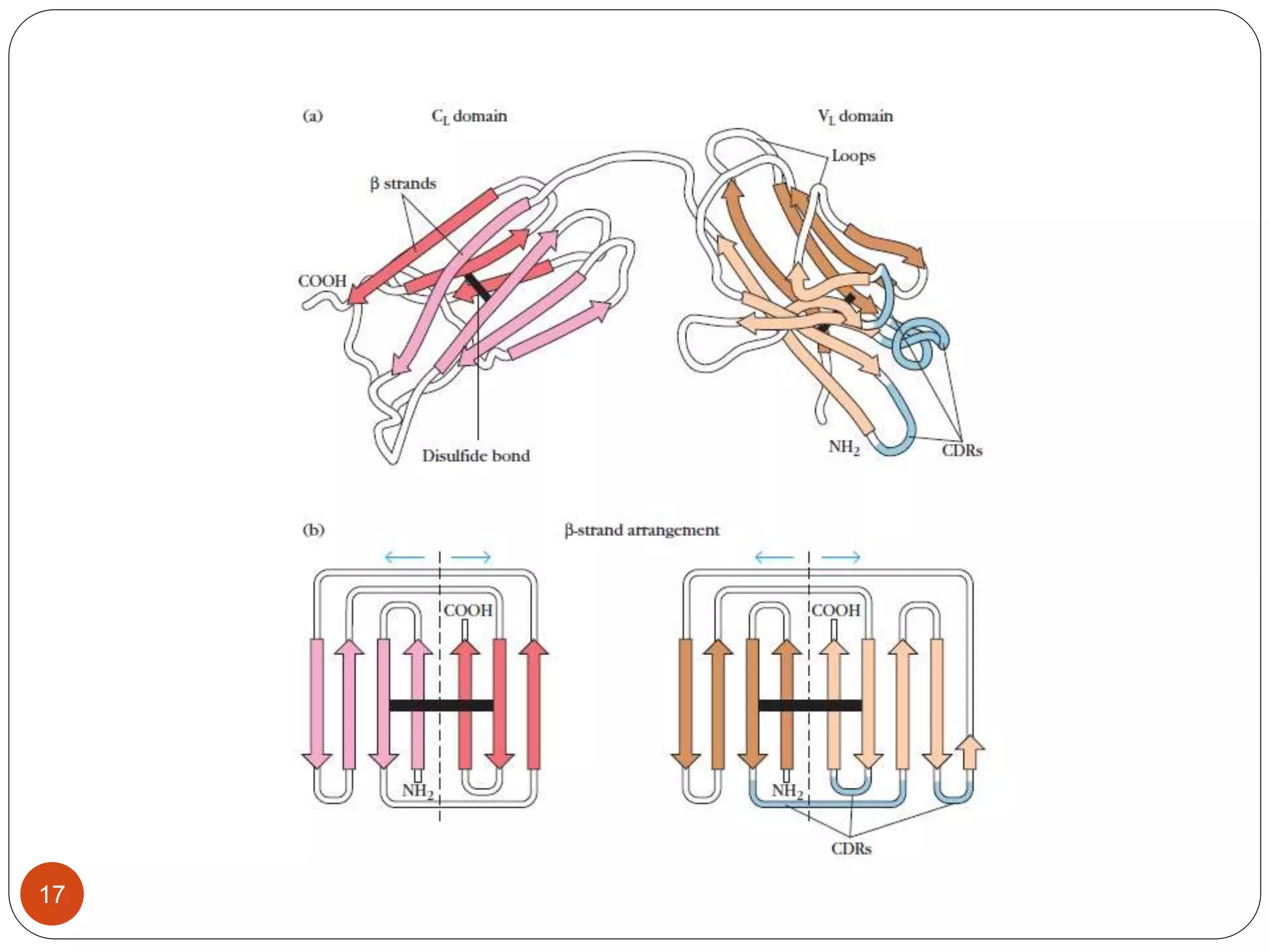
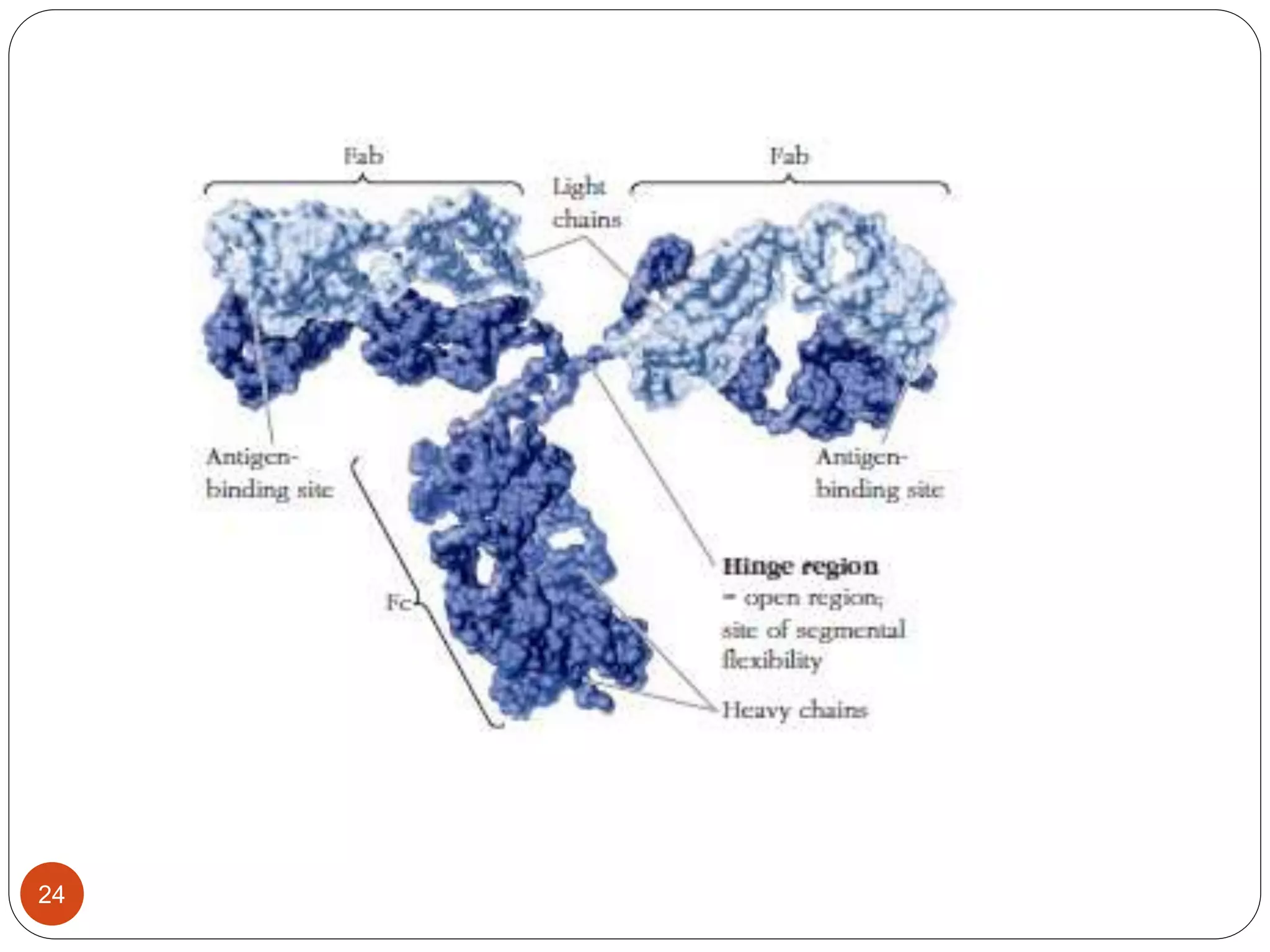
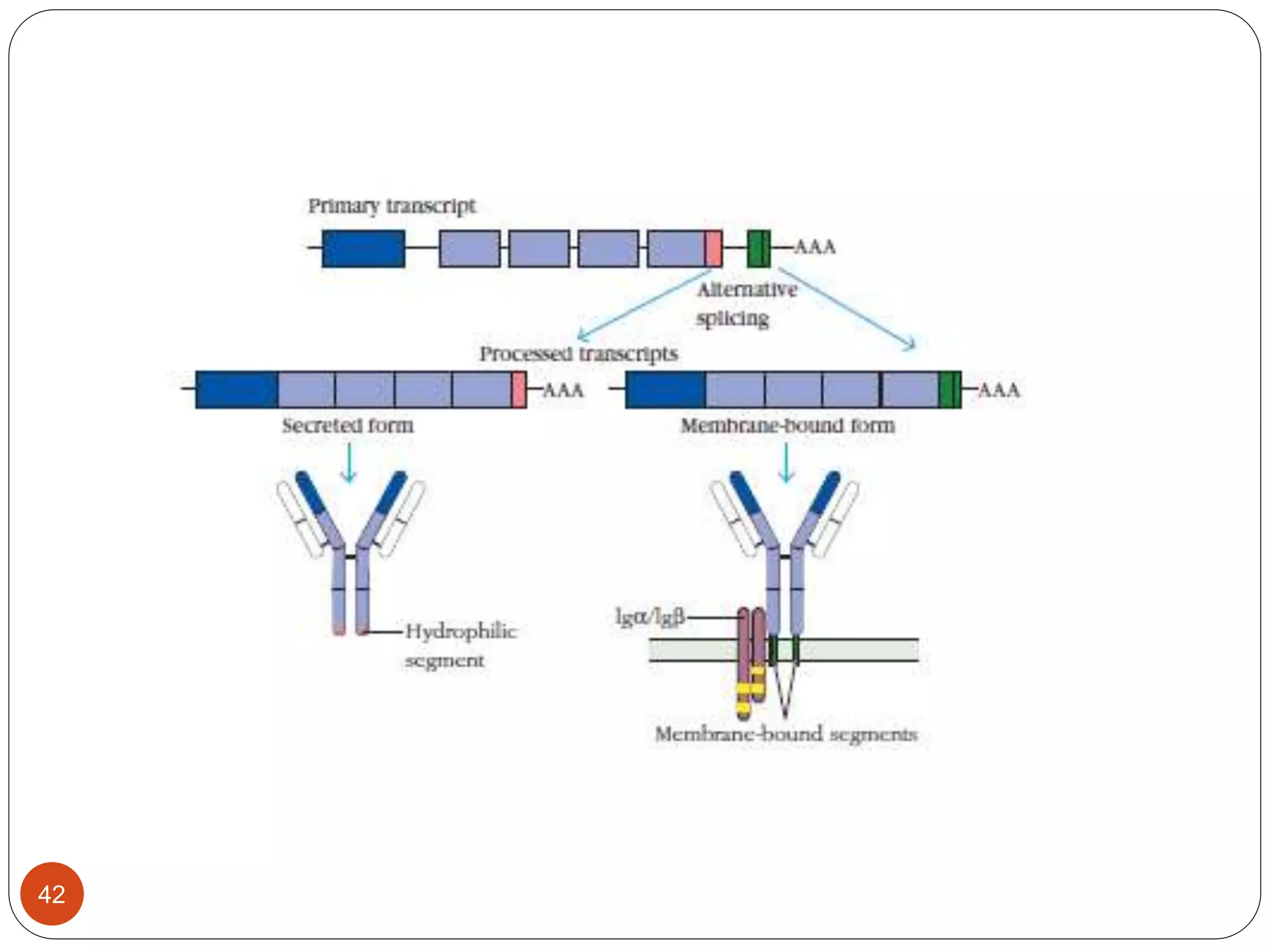
3) Antibodies and receptors share an immunoglobulin domain structure allowing different binding sites to be built into flexible regions. Their Y-shaped structure links antigen binding and immune effector functions.