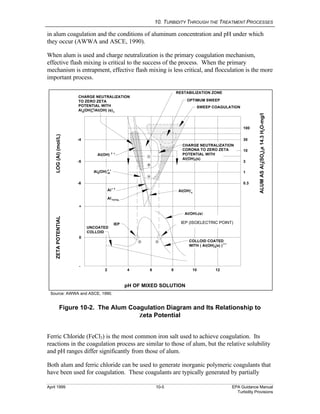
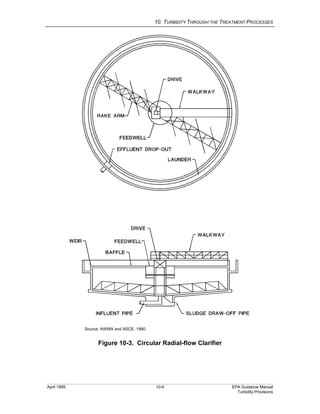
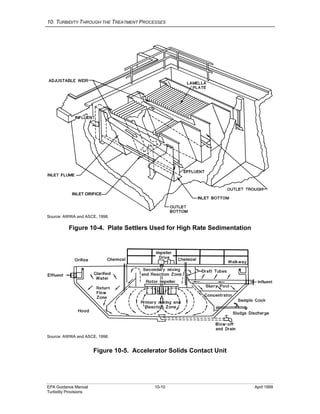
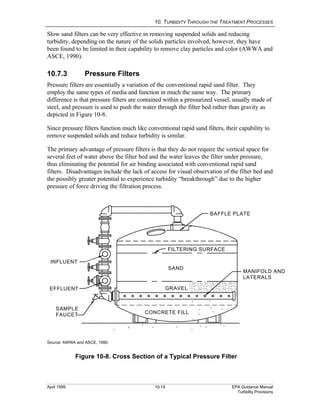
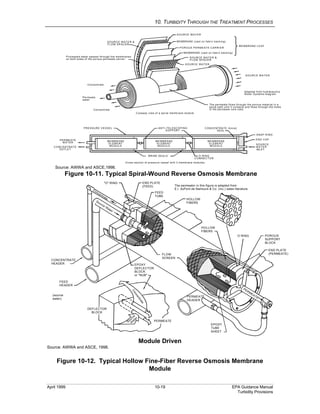
This document provides an overview of various water treatment processes and their effects on turbidity. It describes how intake facilities can screen out large debris but have little effect on turbidity. Pre-sedimentation basins and chemical addition can significantly reduce turbidity over longer detention times. Coagulation destabilizes particles for removal but does not reduce turbidity on its own. Flocculation agglomerates destabilized particles to facilitate their removal through sedimentation and filtration, ultimately reducing turbidity.