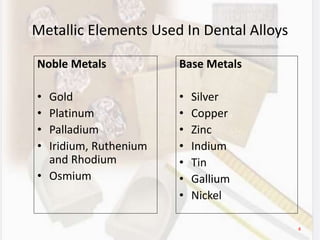
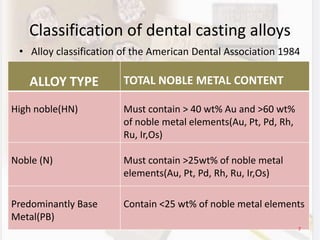
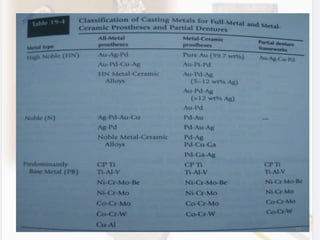
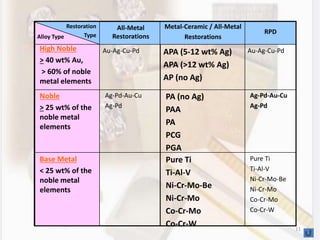
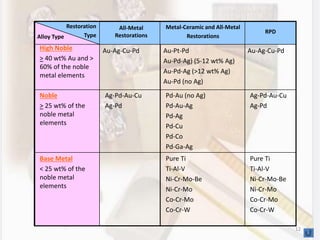
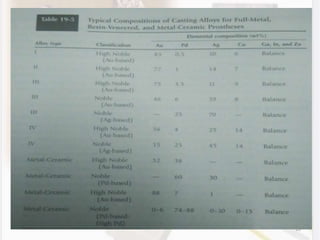
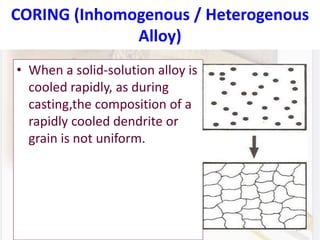
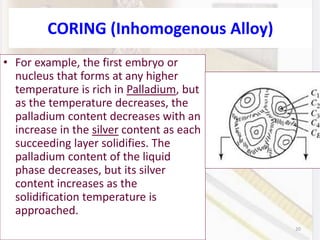
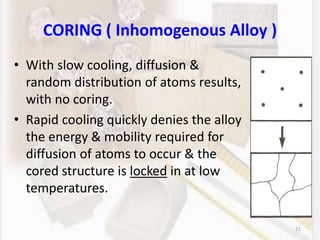
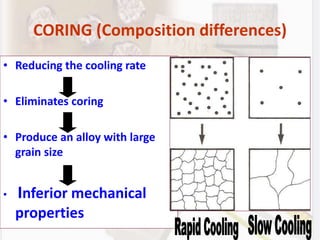
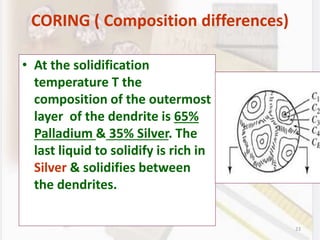
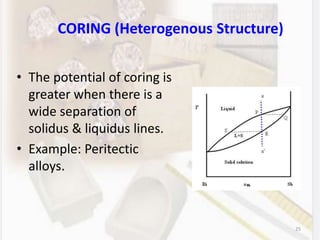
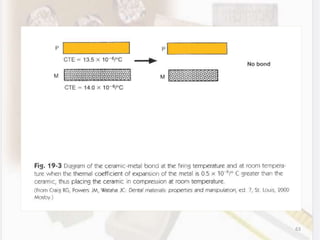
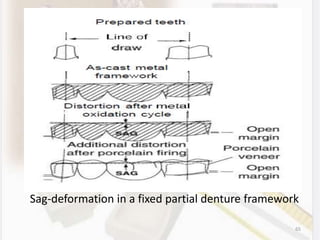
This document discusses various aspects of casting alloys used in dentistry. It defines key terminology related to alloy composition and properties. Common metallic elements used in dental alloys are categorized as noble metals or base metals. Desirable properties for dental casting alloys include biocompatibility, corrosion resistance, and strength requirements. Alloys are classified based on their total noble metal content. Common heat treatments for dental alloys like homogenization, softening, and hardening are explained. Common alloys used for all-metal and metal-ceramic restorations are highlighted.