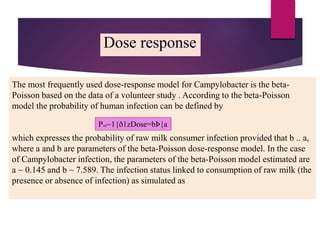
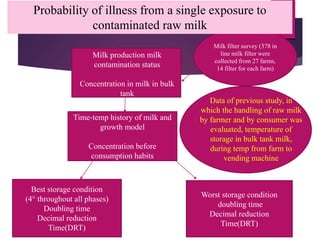
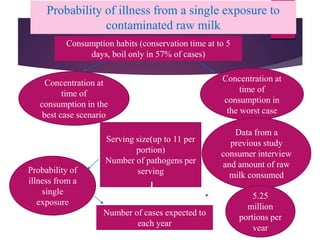
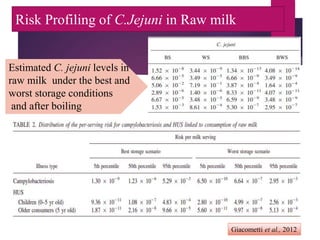
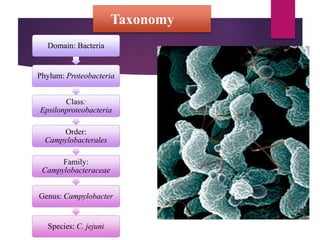
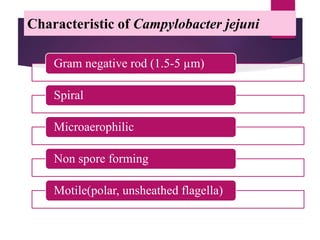
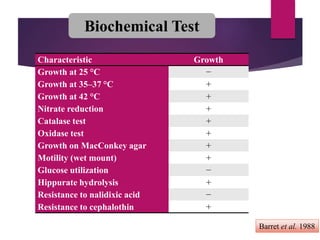
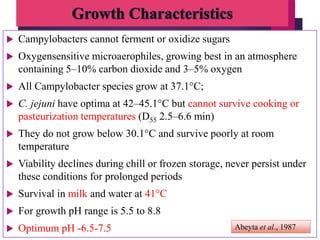
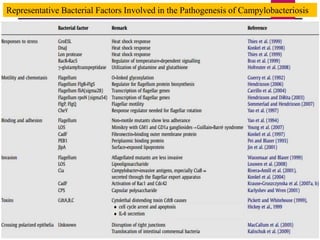
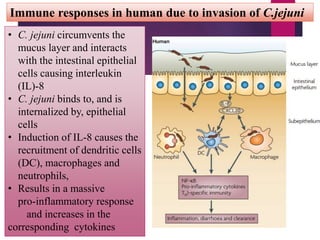
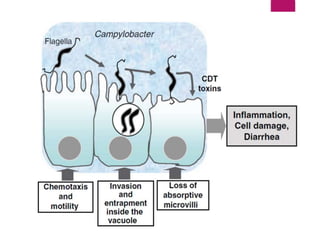
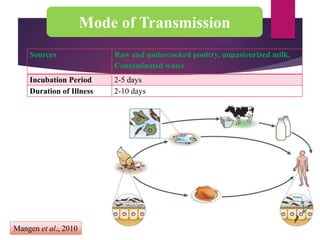
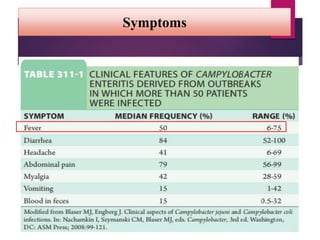
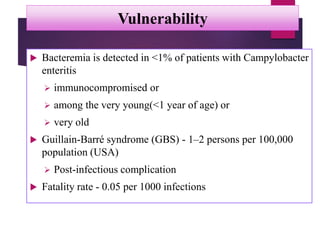
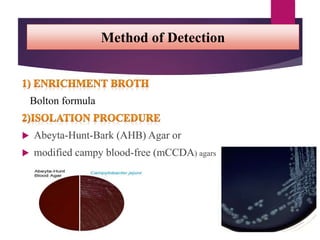
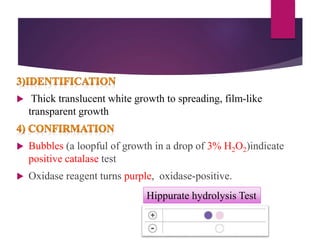
Campylobacter jejuni is a significant zoonotic pathogen associated with foodborne illnesses, particularly from raw milk and undercooked poultry, causing campylobacter enteritis. The bacteria thrive in specific temperature and pH conditions, with survival rates declining during chilling and prolonged storage, and can lead to serious complications like Guillain-Barré syndrome in vulnerable populations. Risk assessments indicate the presence of C. jejuni in various milk products, highlighting the importance of pasteurization and safe food handling practices to mitigate potential outbreaks.






![For each pathogen considered, two dose output models
were achieved:
one for the best and the other for the worst storage milk
chain scenarios
Dose Campylobacter40C
~10˄ (C-[DRT(Camp 4°)X T(h)]-Boil) x Si
Dose CampylobacterDT
~10˄ (C-DRT(Camp ΔTX T(h)]-Boil) x Si
Pathogen dose per serving size](https://image.slidesharecdn.com/c-220625210427-4dbe8d42/85/c-jejuni-pptx-28-320.jpg)