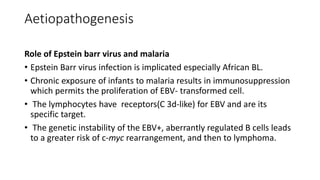
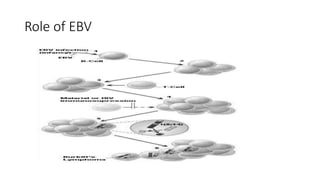
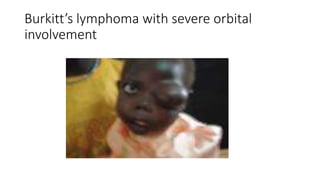
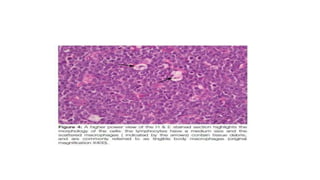
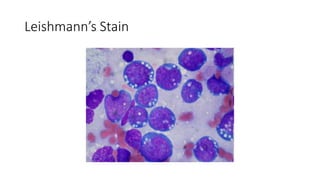
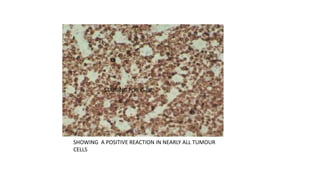
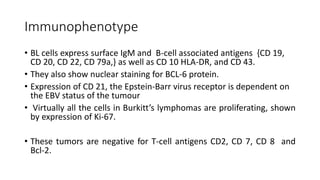
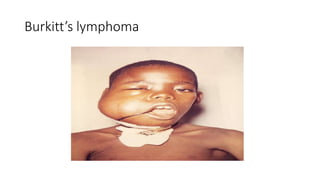
Burkitt's lymphoma is a highly aggressive B-cell lymphoma characterized by a translocation of the c-myc gene. It typically presents as a rapidly growing abdominal or facial tumor in children. Treatment involves intensive short-duration chemotherapy, which can cure over 80% of cases of the endemic form. Prognosis is generally good if the cancer is localized and responds well to chemotherapy, but patients presenting with widespread or refractory disease have a worse outcome.














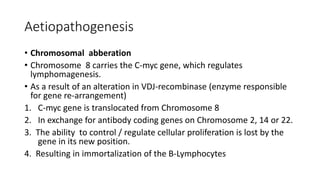
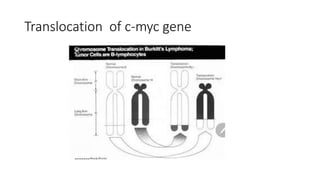
![• Three alterations have been described
• Translocation resulting in the transposition of the C-myc proto-
oncogene on chromosome 8 with one of the immunoglobulin heavy
chain (IgH) genes on chromosome 14. [t(8;14)(q24;q32) ].
• This occurs in 60-70% of cases.](https://image.slidesharecdn.com/burkittlymphoma-230518122954-e23bfd4d/85/Burkitt-lymphoma-pptx-21-320.jpg)
![• c-myc on chromosome 8 translocated close to the λ-light-chain gene
on chromosome 22 [t(8;22)(q24;q11)]. 10-15% of cases.
• c-myc on chromosome 8 translocated close to the ƙ-light-chain gene
on chromosome 2 [t(8;2)(p12;q24)]. 2-5% of cases.
• These result in overexpression of the c-myc gene and is considered
responsible for tumor proliferation.](https://image.slidesharecdn.com/burkittlymphoma-230518122954-e23bfd4d/85/Burkitt-lymphoma-pptx-22-320.jpg)