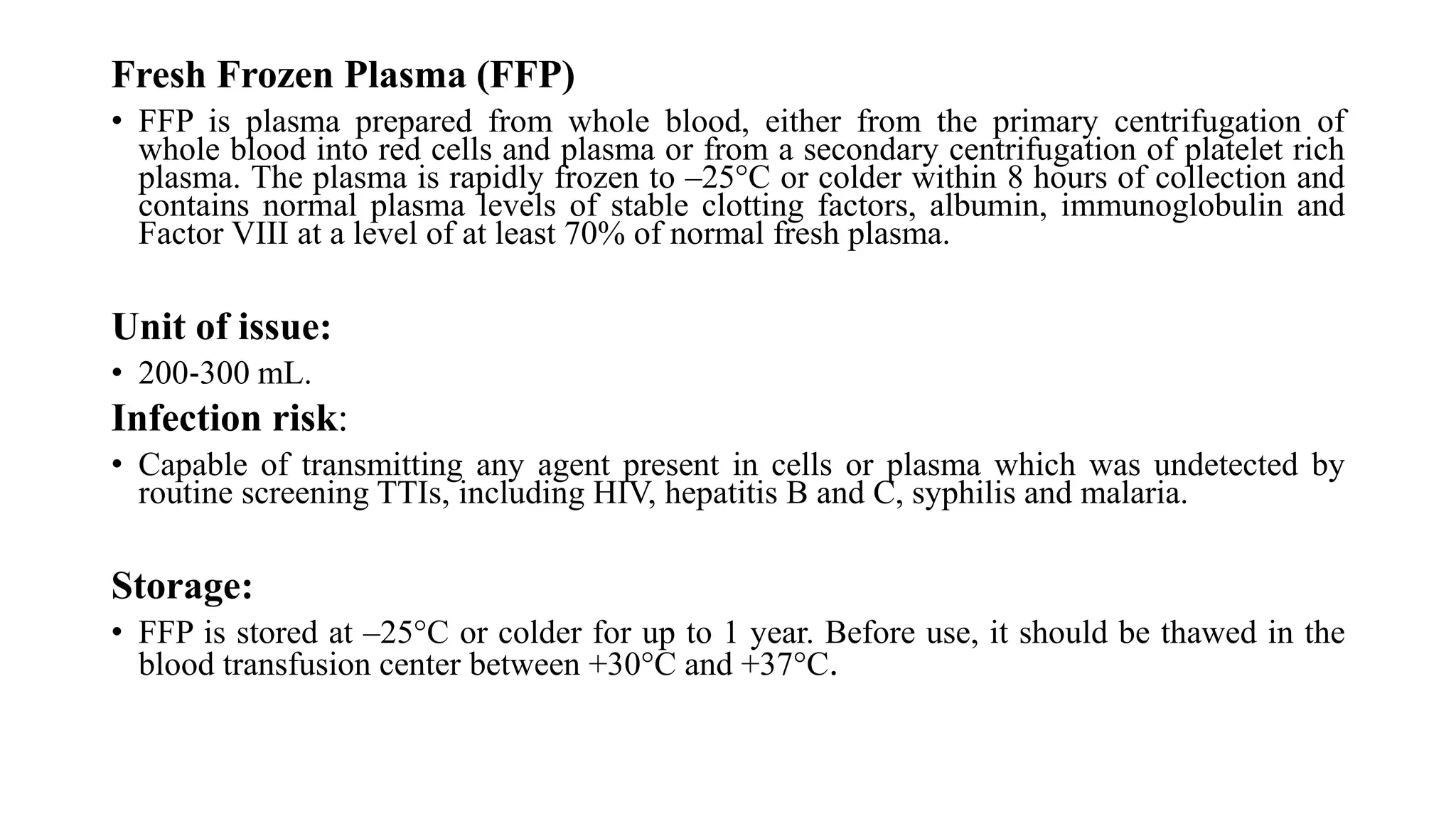
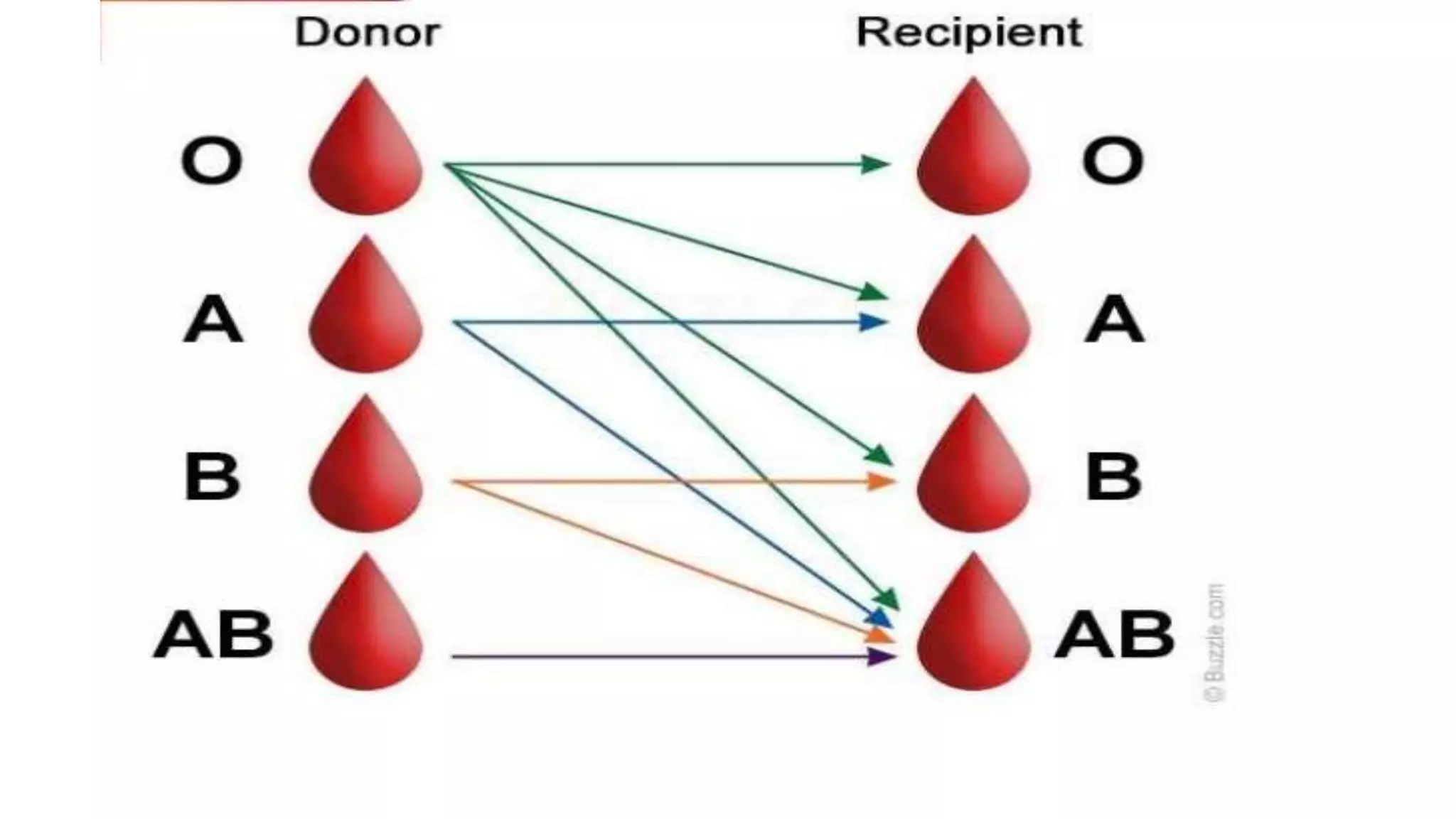
This document discusses blood groups and blood transfusions. It begins by describing red blood cells and their functions. It then covers the different blood types (A, B, AB, and O) based on the presence or absence of antigens, and the corresponding circulating antibodies. The document discusses blood typing, transfusion compatibility, and the importance of screening donors and testing blood. It explains components of blood that can be transfused including red blood cells, plasma, platelets, and cryoprecipitate. The goal of blood transfusion is to provide safe and adequate blood products to meet patients' needs.





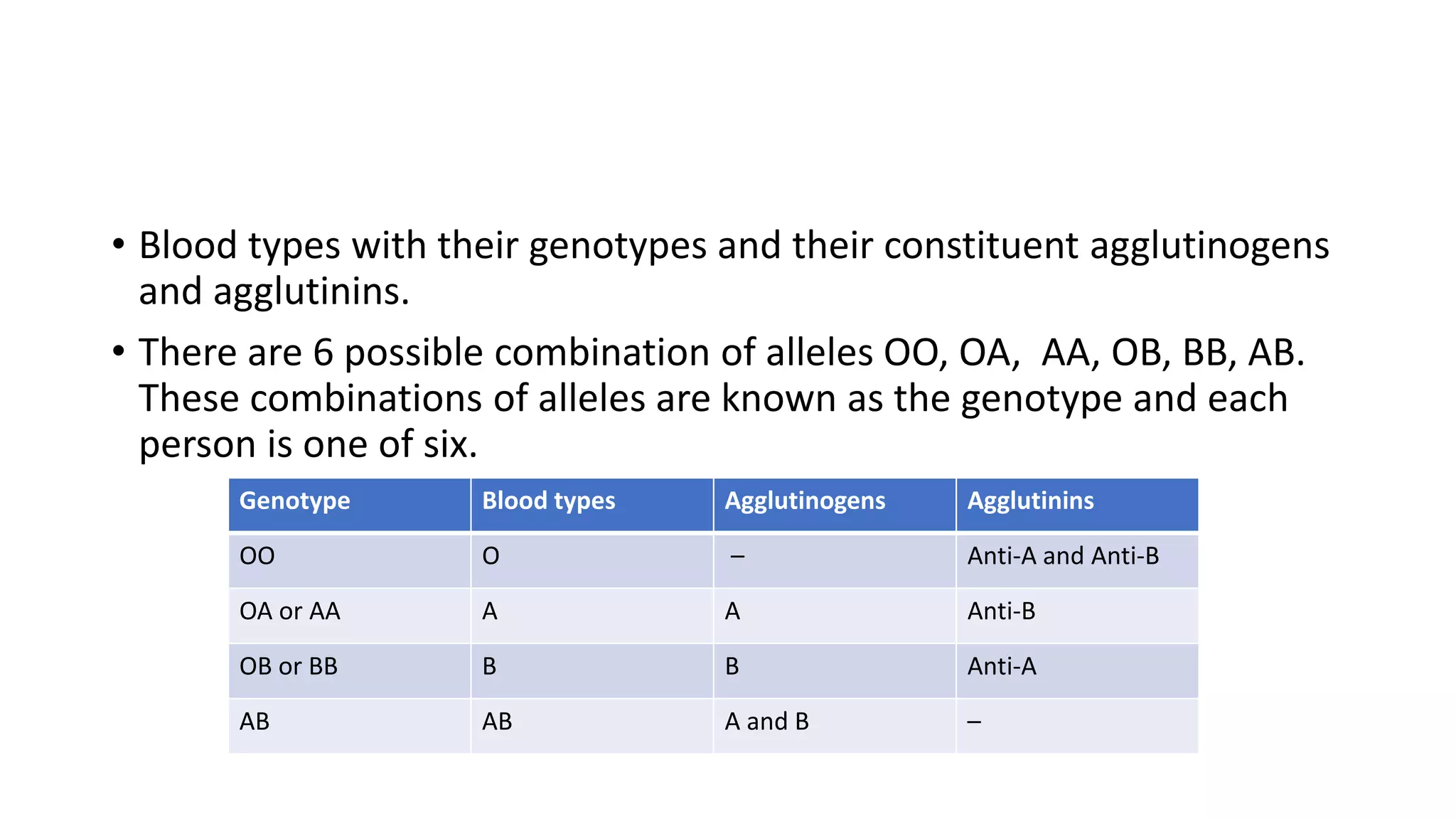

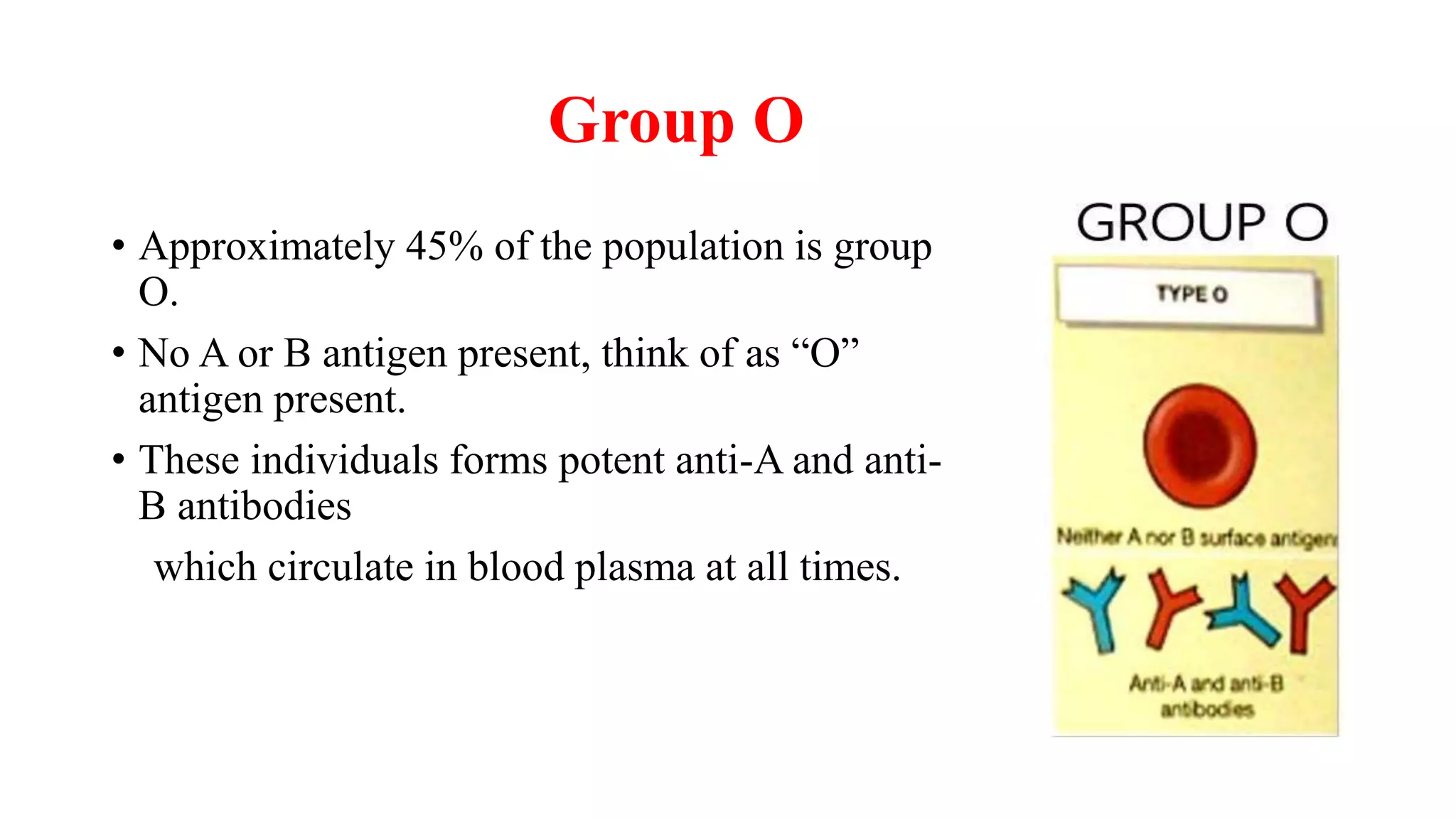





















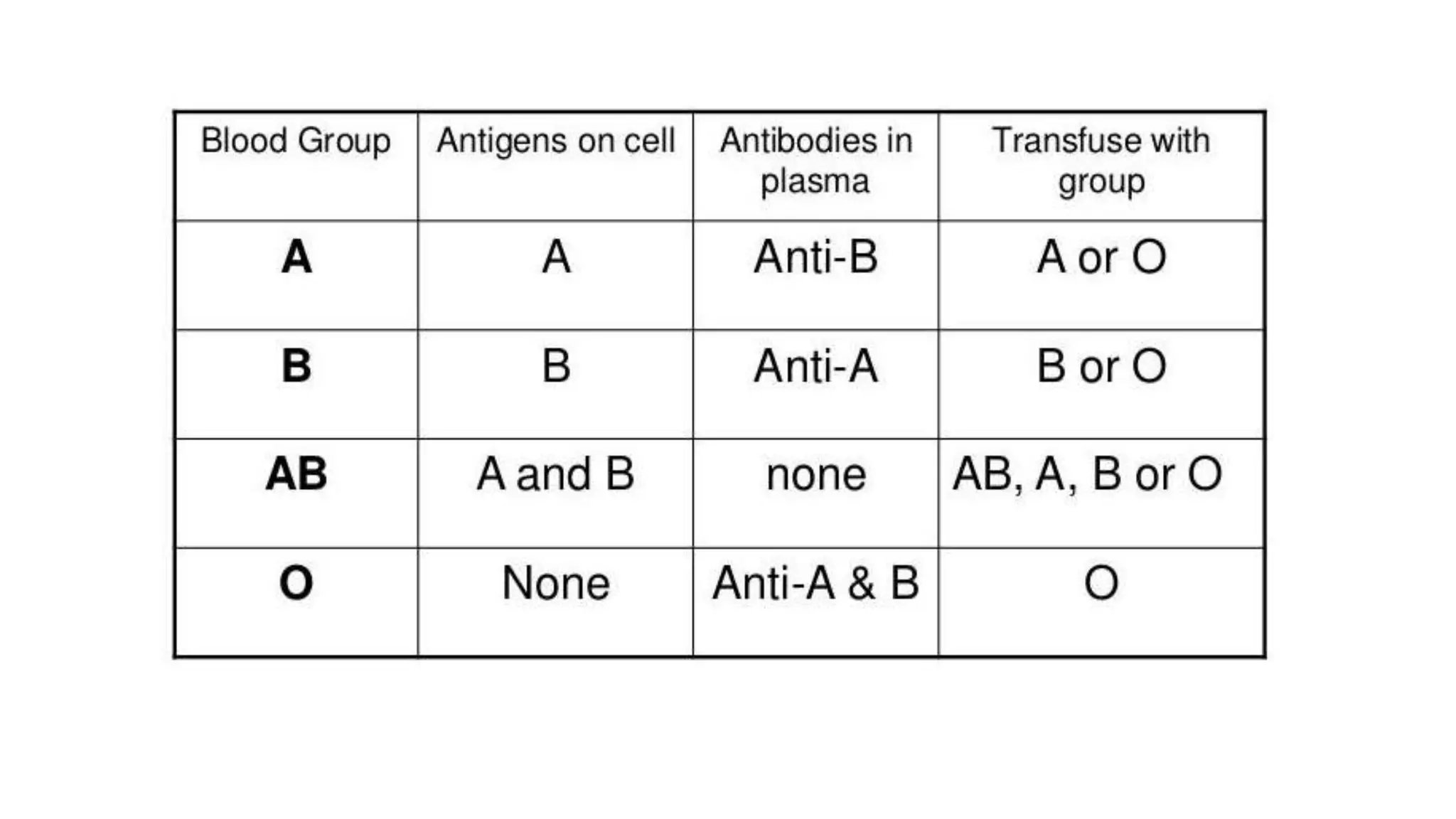


![Red cell concentrates [packed red blood cells (PRBC)]
• 150‐200 mL red blood cells from which most of the plasma has been removed. Hb concentration
will be approximately 20 g/100 mL (not less than 45 g per unit) and Hct 55‐75%.
Storage:
• Between +2°C and +6°C in an approved blood bank refrigerator, fitted with a temperature monitor
and alarm.
Indications: Replacement of red cells in anaemic patients.](https://image.slidesharecdn.com/bloodgroupsandbloodtransfusions-220909162205-2bb06e13/75/BLOOD-GROUPS-AND-BLOOD-TRANSFUSIONS-pptx-34-2048.jpg)