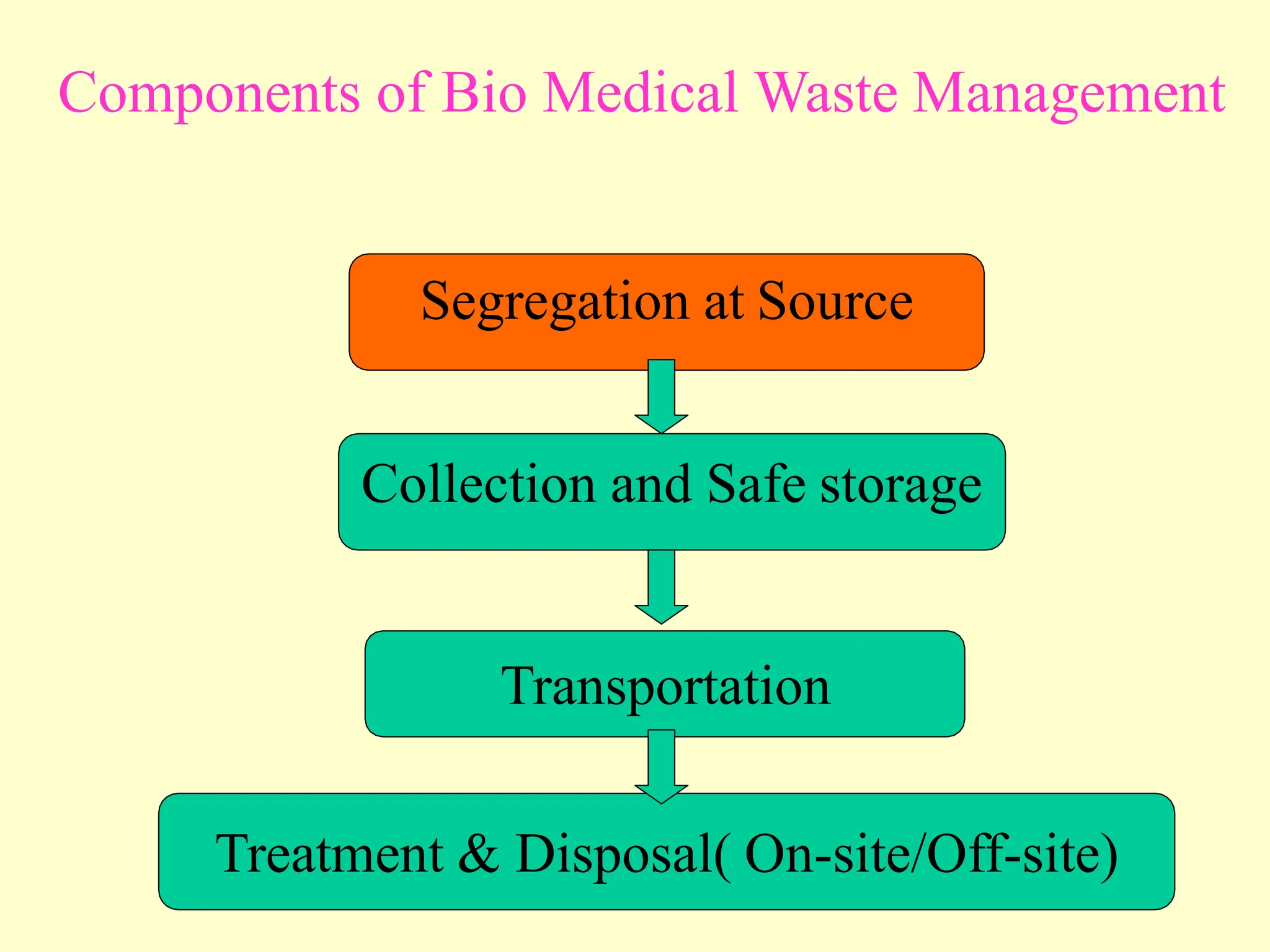
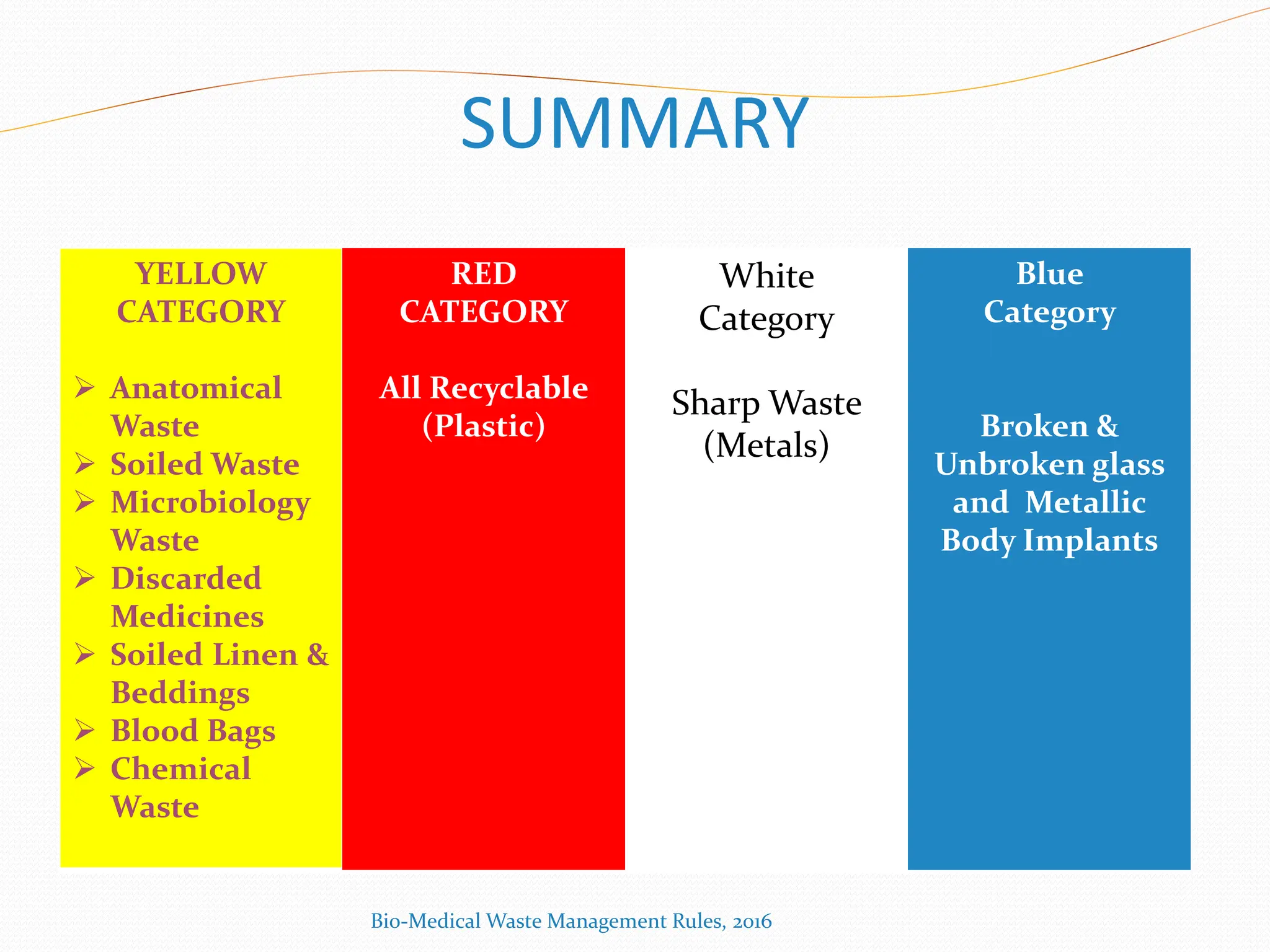
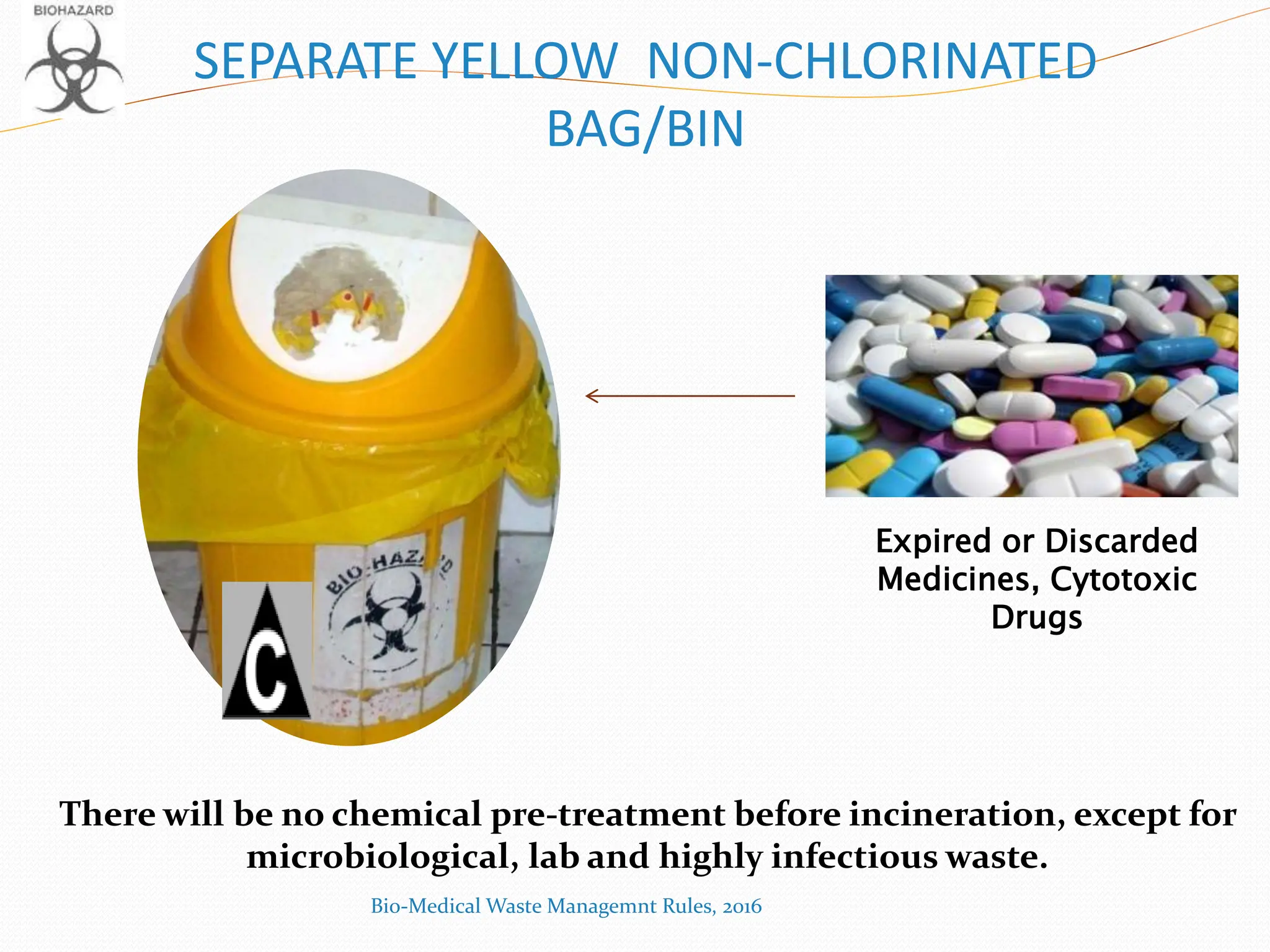
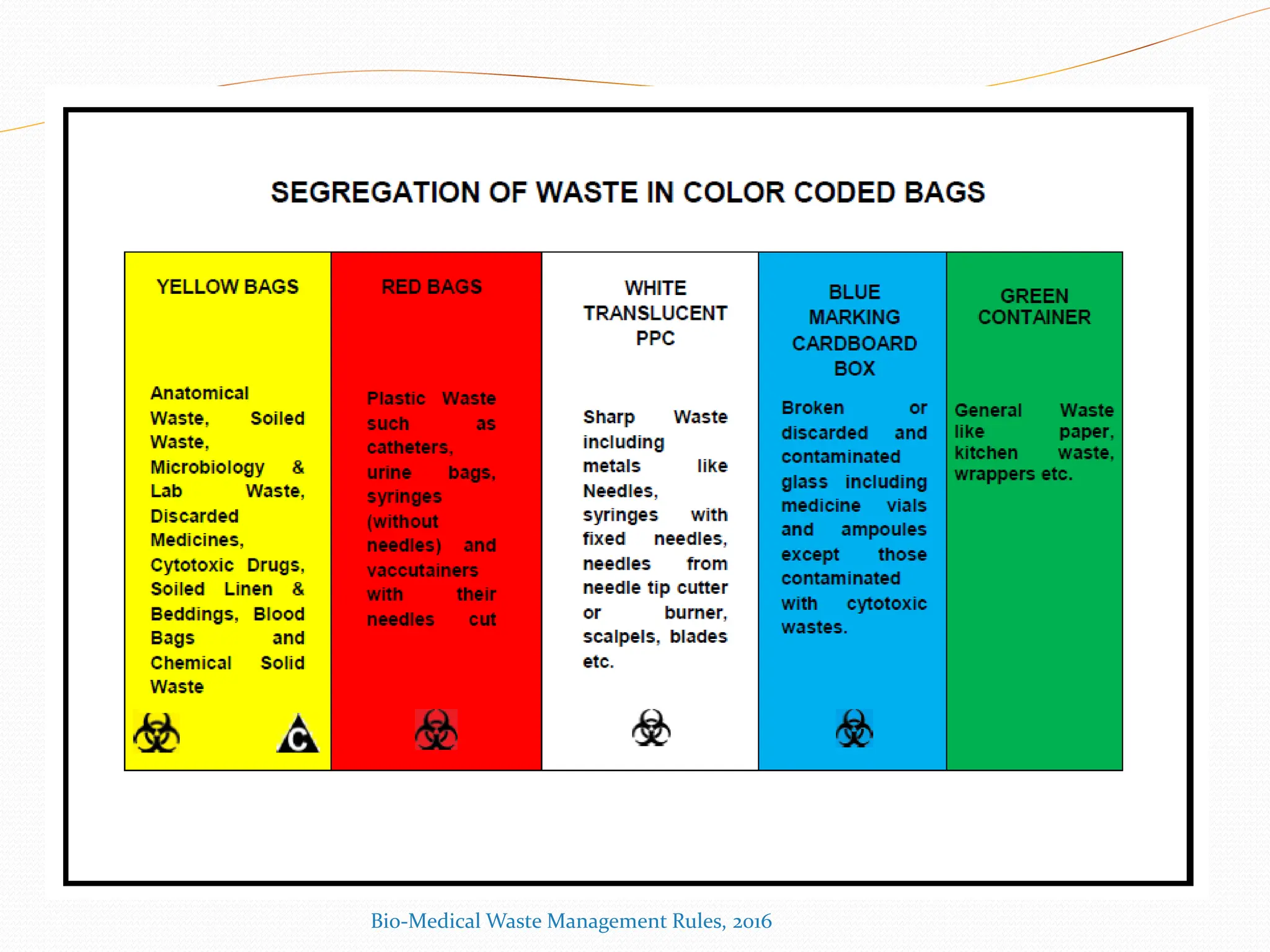
The Bio-Medical Waste Management Rules, 2016 govern the segregation, collection, treatment, and disposal of bio-medical waste generated by healthcare facilities in India. Key provisions include responsibilities for waste management, color coding systems for waste segregation, and the establishment of waste management committees in hospitals. Compliance with these rules is crucial to prevent hazardous and infectious waste from contaminating non-infectious waste and to ensure environmental protection.