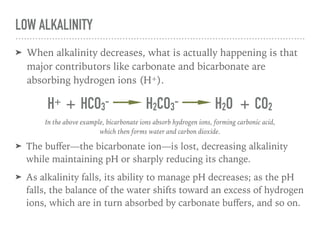
This document discusses alkalinity demand in swimming pools. It explains that total alkalinity measures the buffering capacity of pool water. Buffers like carbonate and bicarbonate ions help maintain pH levels by absorbing hydrogen or hydroxide ions. When alkalinity decreases, these buffers are consumed, reducing their ability to regulate pH. Once pH falls below 5, alkalinity is effectively zero and is said to be in demand. To treat alkalinity demand, pH should first be raised above 5 before adding more buffers to raise alkalinity levels. Regular testing and adjustment of both pH and alkalinity can prevent alkalinity demand from occurring.