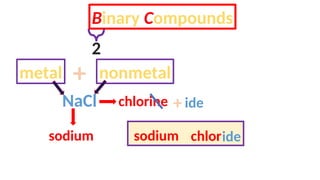
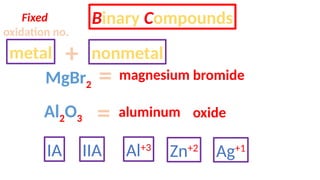
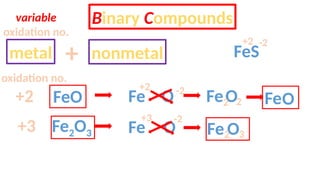
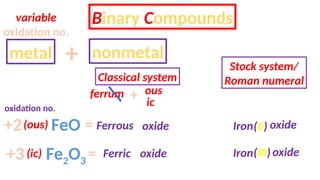
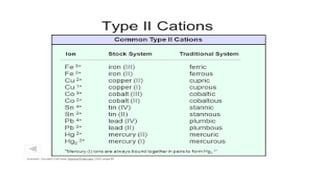
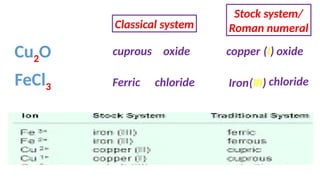
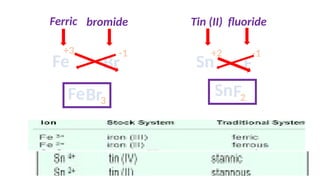
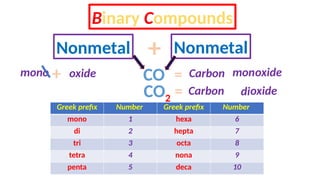
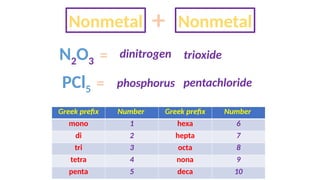
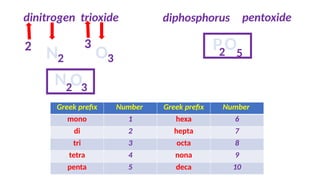
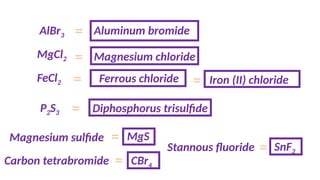
The document discusses the nomenclature of inorganic compounds, focusing on binary compounds comprised of metals and nonmetals, including fixed and variable oxidation states. It outlines the classical and stock systems for naming these compounds, providing examples such as ferrous and ferric oxides, as well as the use of Greek prefixes in naming nonmetal compounds. Additionally, it lists various chemical compounds with their corresponding formulas and naming conventions.